COVID-19: China Medical Supply Chains and Broader Trade Issues
Changes from April 6, 2020 to October 8, 2020
This page shows textual changes in the document between the two versions indicated in the dates above. Textual matter removed in the later version is indicated with red strikethrough and textual matter added in the later version is indicated with blue.
COVID-19: China Medical Supply Chains and Broader Trade Issues
Contents
- Overview
- U.S.-China Trade and the Impact of COVID-19
- China First Quarter (Q1) 2020 Slowdown Effects on U.S. Industries
- Transportation, Logistics and Broader Considerations
- Prospects for U.S. Exports
- Force Majeure Provisions
- U.S. Reliance on China for Health Care and Medical Products
- China Nationalizes Medical Production and Supply
- Implications of China's Export Constraints: U.S. Shortages and Policy Response
- Global Trade Restrictions
- China's Economic Recovery: Prospects and Implications
- China Positioning to Export
- Steel Overcapacity
- Export VAT Rebate
- China Pushing Ahead in Strategic Sectors
- Issues for Congress
- Dependency of U.S. Health Care Supply Chains on China
- Other U.S. Supply Chain Dependencies
- U.S. Market Competitiveness and Tariff Policy
- Information and Data Gaps
- Unique Role of the U.S. Federal Government
- U.S. Leadership on Global Trade and Health Issues
Figures
Tables
- Table 1. Select U.S. Imports from China in 2019
- Table 2. Change in China's Exports and Imports of Select Medical Products
- Table 3. U.S. Imports from China in 2019: COVID-19 Related Medical Supplies
- Table 4. Recent Section 301 Tariff Exclusions on Select U.S. Imports from China
- Table 5. China's Export VAT Rebates, March 2020 (9%-13%)
- Table B-1. Select U.S. Import and Exports in 2019
- Table B-2. U.S. Imports of Pharmaceuticals and Medical Equipment, Products, and Supplies in 2019
Summary
COVID-19: China Medical Supply Chains and October 8, 2020 Broader Trade Issues Karen M. Sutter, The outbreak of Coronavirus Disease 2019 (COVID-19), first in China, and then Coordinator globally, including in the United States, is drawing attention to the ways in which the Specialist in Asian Trade U.S. economy depends on manufacturing and supply chains based in China. This report and Finance aims to assess current developments and identify immediate and longer range China trade issues for Congress.
Andres B. Schwarzenberg Analyst in International
An area of particular concern to Congress is U.S. shortages in medical supplies—
Trade and Finance
including personal protective equipment (PPE) and pharmaceuticals—as the United
States steps up efforts to contain COVID-19 with limited domestic stockpiles and
Michael D. Sutherland
insufficient U.S. industrial capacity. Because of China'’s role as a global supplier of PPE,
Analyst in International
medical devices, antibiotics, and active pharmaceutical ingredients, reduced export exports
Trade and Finance
from China have led to shortages of critical medical supplies in the United States.
Exacerbating the situation, in early February 2020, the Chinese government nationalized
control of the production and distribution of medical supplies in China—directing all production for domestic use—and directed the bureaucracy and Chinese industry to secure supplies from the global market. Now apparentlyOnce past the initial peak of its COVID-19 outbreak, the Chinese government may selectively releaseappears to have prioritized certain countries and selectively released some medical supplies for overseas delivery, with designated countries selected, according to political calculations.
.
Congress has enacted legislation to better understand and address U.S. medical supply chain dependencies, including P.L. 116-136, The Coronavirus Aid, Relief, and Economic Security (CARES) Act, that includes several provisions to:
- expand drug shortage reporting requirements;
- require certain drug manufacturers to draw up risk management plans;
- require the U.S. Food and Drug Administration (FDA) to maintain a public list of medical devices
that are determined to be in shortage; and
-
direct the National Academies of Science, Engineering, and Medicine to conduct a study of
pharmaceutical supply chain security.
Other potential considerations for Congress include whether and how to further incentivize additional production of health supplies, diversify production, address other supply chain dependencies (e.g., microelectronics), fill information and data gaps, and promote U.S. leadership on global health and trade issues.
The crisis that has emergedmerged for the U.S. economy is defined, in large part, by a collapse of critical supply, as well as a sharp downturn in demand, first in China and now in the United States and globally. As China'’s manufacturing sector recovers, while the United States and other major global markets are grappling with COVID-19, some fear China could overwhelm overseas markets, as it ramps up export-led growth to compensate for the sharp downturn of exports in the first quarter of 2020, secure hard currency, and boost economic growth. China may also seek to make gains in strategic sectors—such as telecommunications, microelectronics, and semiconductors—in which the government undertook extraordinary measures to sustain research and development and manufacturing during the COVID-19 outbreak in China.
Overview
The outbreak of coronavirus disease (COVID-19), first in the People'
Congressional Research Service
link to page 5 link to page 6 link to page 8 link to page 10 link to page 12 link to page 13 link to page 13 link to page 17 link to page 22 link to page 28 link to page 32 link to page 34 link to page 37 link to page 38 link to page 39 link to page 40 link to page 41 link to page 45 link to page 46 link to page 50 link to page 50 link to page 51 link to page 53 link to page 54 link to page 8 link to page 14 link to page 14 link to page 17 link to page 24 link to page 25 link to page 40 link to page 42 link to page 15 link to page 19 link to page 20 COVID-19: China Medical Supply Chains and Broader Trade Issues
Contents
Overview ......................................................................................................................................... 1 U.S.-China Trade and the Impact of COVID-19 ............................................................................. 2
China First Quarter (Q1) 2020 Slowdown Effects on U.S. Industries ...................................... 4 Transportation, Logistics and Broader Considerations ............................................................. 6 Prospects for U.S. Exports ........................................................................................................ 8
Force Majeure Provisions ................................................................................................... 9
U.S. Reliance on China for Health Care and Medical Products ...................................................... 9
China Nationalizes Medical Production and Supply ............................................................... 13 Implications of China’s Export Constraints: U.S. Shortages and Policy Response ................ 18
U.S. Shortages ................................................................................................................... 24
Global Trade Restrictions ........................................................................................................ 28
Domestic Supply: U.S. vs Foreign Made Products ........................................................... 30
China’s Economic Recovery: Prospects and Implications ............................................................ 33
China Positioning to Export .................................................................................................... 34 Steel Overcapacity .................................................................................................................. 35 Export VAT Rebate .................................................................................................................. 36 China Pushing Ahead in Strategic Sectors .............................................................................. 37
Issues for Congress ........................................................................................................................ 41
Dependency of U.S. Health Care Supply Chains on China .................................................... 42 Other U.S. Supply Chain Dependencies ................................................................................. 46 U.S. Market Competitiveness and Tariff Policy ...................................................................... 46 Information and Data Gaps ..................................................................................................... 47 Unique Role of the U.S. Federal Government ........................................................................ 49 U.S. Leadership on Global Medical Trade .............................................................................. 50
Figures Figure 1. U.S.-China Trade in 2019 ................................................................................................ 4 Figure 2. U.S. Imports of Pharmaceuticals and Medical Equipment, Products, and
Supplies in 2019 ......................................................................................................................... 10
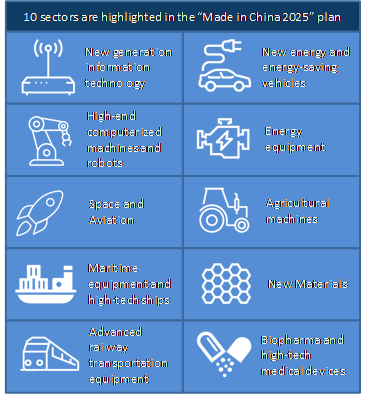
Figure 3. U.S. Import and Exports of Select Medical Products in 2019 ....................................... 13 Figure 4. China’s Export of Select Covid-19-Related Products: Jan.-July 2020 .......................... 20 Figure 5. China’s Exports of Select Covid-19-Related Products: ................................................. 21 Figure 6. China Raw Steel Production (2000-2019) ..................................................................... 36 Figure 7. China’s Industrial Priorities (2015-2025) ...................................................................... 38
Tables Table 1. Select U.S. Imports from China in 2019 ........................................................................... 11 Table 2. Change in China’s Exports and Imports of Select Medical Products .............................. 15 Table 3. U.S. Imports from China in 2019: COVID-19 Related Medical Supplies ...................... 16
Congressional Research Service
link to page 25 link to page 25 link to page 30 link to page 35 link to page 35 link to page 41 link to page 56 link to page 56 link to page 56 link to page 56 COVID-19: China Medical Supply Chains and Broader Trade Issues
Table 4. Top Partners: Value of China’s Exports of Select Covid-19-Related Medical
Goods ......................................................................................................................................... 21
Table 5. Recent Section 301 Tariff Exclusions on Select U.S. Imports from China ..................... 26 Table 6. Estimate of the Imported Share of U.S. Domestic Supply: Select Medical-
Related Manufactured Good Categories in 2018 ....................................................................... 31
Table 7. China’s Export VAT Rebates, March 2020 (9%-13%) .................................................... 37
Table A-1. U.S. Imports of Pharmaceuticals and Medical Equipment, Products, and
Supplies in 2019 ......................................................................................................................... 52
Appendixes Appendix A. U.S. Imports of Select Medical Products ................................................................. 52
Contacts Author Information ........................................................................................................................ 52
Congressional Research Service
COVID-19: China Medical Supply Chains and Broader Trade Issues
Overview The outbreak of Coronavirus Disease 2019 (COVID-19), first in the People’s Republic of China (PRC or China), and now globally, including in the United States, is drawing attention to the ways in which the United States and other economieseconomies depend on critical manufacturing and global value chains that rely on production based in China. Congress is particularly concerned about these dependencies and has passed legislation to better understand and address them. An area of particular concern to Congress in the current environment is U.S. shortages of medical supplies—including personal protective equipment (PPE) and pharmaceuticals—as the United States steps up efforts to contain COVID-19 with limited domestic stockpiles and insufficient U.S. industrial capacity. Because of China'’s role as a global supplier of PPE, medical devices, antibiotics, and active pharmaceutical ingredients (API), reduced exports from China have led to shortages of critical medical supplies in the United States.1
1
Starting in early February 2020, U.S. health care experts began warning of a likely global spread of COVID-19, and early reports of U.S. medical supply shortages began to emerge. At the same time, the Chinese government nationalized control of the production and distribution of medical supplies in China, directing all production for domestic use.22 The Chinese government also directed the national bureaucracy, local governments, and Chinese industry to secure supplies from the global market.33 This effort likely exacerbated medical supply shortages in the United States and other countries, particularly in the absence of domestic emergency measures that might have locked in domestic contracts, facilitated an earlier start to alternative points of production, and restricted exports of key medical supplies. As China'’s manufacturing sector recovers while the United States and other countries are grappling with COVID-19, the Chinese government may selectively release some medical supplies for overseas delivery. Those decisions are likelyprioritize certain countries for overseas delivery of medical supplies. Those decisions appear to be driven, at least in part, by political calculations, as it has done recently with many countries around the world.4
4
1 Finbarr Bermingham and Su-Lin Tan, “Coronavirus: China’s mask-making juggernaut cranks into gear, sparking fears of over-reliance on world’s workshop,” South China Morning Post, March 12, 2020, https://www.scmp.com/economy/global-economy/article/3074821/coronavirus-chinas-mask-making-juggernaut-cranks-gear; U.S. Food and Drug Administration, “Coronavirus (COVID-19) Supply Chain Update,” Press release, February 27, 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update.
2 Zhang Pinghui and Zhou Xin, “Coronavirus: China Shifts Responsibility Over Medical Supplies Amid Mask Shortage, Rising Death Toll,” South China Morning Post, February 3, 2020, updated on February 14, 2020, https://www.scmp.com/economy/china-economy/article/3048744/coronavirus-mask-shortage-prompts-beijing-tweak-authority; Finbarr Bermingham and Su-Lin Tan, “Coronavirus: China’s Mask Making Juggernaut Cranks Into Gear, Sparking Fears of Overreliance on World’s Workshop,” South China Morning Post, March 12, 2020, https://www.scmp.com/economy/global-economy/article/3074821/coronavirus-chinas-mask-making-juggernaut-cranks-gear; Engen Tham, Cheng Leng, and Zhang Yan, “Exclusive: Unilever, 3M on List of Firms Eligible for China Loans to Ease Coronavirus Crisis—Sources,” Reuters, February 19, 2020, https://www.reuters.com/article/us-china-health-lending-exclusive-idUSKBN20D0SQ; Yang Jian, “GM, Wuling Venture Begins Output of Machines to Make Face Masks, Automotive News, February 20, 2020, https://www.autonews.com/china/gm-wuling-venture-begins-output-machines-make-face-masks; and Luffy Liu, “700 Tech Companies in China Have Begun Making Masks,” EE Times, February 13, 2020, https://www.eetimes.com/700-tech-companies-in-china-have-begun-making-masks/. 3 “Circular on Further Facilitating the Import and Export of Technology During the Period of Epidemic Prevention and Control,” PRC Ministry of Commerce, February 4, 2020, http://english.mofcom.gov.cn/article/newsrelease/significantnews/202002/20200202934774.shtml; and “Circular on Actively Expanding Imports to Combat Against Novel Coronavirus Epidemic,” PRC Ministry of Commerce, February 6, 2020, http://english.mofcom.gov.cn/article/newsrelease/significantnews/202002/20200202.
4 Li Yan, “Xi Says China to Send More Medical Experts to Italy,” Xinhua, March 17, 2020, http://www.ecns.cn/m/news/politics/2020-03-17/detail-ifzunmih1236562.shtml934157.shtml, and “’Mask Diplomacy’
Congressional Research Service
1
COVID-19: China Medical Supply Chains and Broader Trade Issues
COVID-19 was identified in China in December 2019 and peaked in late January 2020. In response, China shut down a large part of its economy in an effort to contain the outbreak. A key factor in the sharp economic slowdown in China was the dramatic downturn of both demand and supply after Chinese officials imposed restrictions in the third week of January on movement of people and goods in and out of localities across China. Since the COVID-19 outbreak in China has eased, the Chinese government'’s efforts to restart business activities has been slow and uneven across sectors and locations. Companies have sought to meet new government requirements for virus containment and faced worker and supply shortages as interregional logistics have remained somewhat constrained.55 Resumption of bilateral trade between the United States and China will likely be uneven due to persistent bottlenecks in inputs, locations of containersthe location of container shipments, and logjams in current shipments. U.S. companies typically maintain anywhere from two to ten weeks of inventory, and transportation time for trans-Pacific container shipments is typically three weeks. With this timeframe in mind, initial shortages that U.S. firms faced of deliveries of microelectronics, auto parts, and health and medical products could intensify over the next few months. Thereintensified once inventory was depleted. Depending on the trajectory of the virus, there could be additional shortages in a wide range of imports that transit via container ship (e.g., processed raw materials, intermediate industrial goods, and finished consumer products).
As China'’s economic activities resume, other countries around the world are taking an economic hit. As in China, new restrictions around the world on the movement of people and business operations could triggerprolong sharp new slowdowns in demand, transportation, and logistics worldwide, further dragging down prospects for global trade recovery. Suppressed global demand will likely further complicate efforts to orchestrate a rebound in China's (or global) economic activityeconomic activity in China (or the world). In sectors where China has extensive capacity (maintains excess capacity, such as steel), some fear China could overwhelm overseas markets, as it ramps up export-led growth to compensate for the sharp economic downturn in the first quarter of 2020.
Congress faces current choices that will influence the longer-range U.S. trade trajectory vis-a-vis China. Since the imposition of Section 301 tariffs on U.S. imports from China and China's ’s retaliatory tariffs beginning in 2018, some Members have raised questions about the dependence of U.S. supply chains on China for critical products. There are also concerns some have raised about the potential ramifications of these dependencies, particularly in times of crisis or PRC nationalization of industry. Current demand pressures during the COVID-19 pandemic could increase U.S. reliance on certain medical supplies from China, at least in the short term (provided that the Chinese government is willing to export these supplies to the United States). At the same time, these pressures are also incentivizing diversification efforts.
as governments and firms re-evaluate the risks of basing substantial portions of their supply chains in China. U.S.-China Trade and the Impact of COVID-19
As the United States'’ third-largest trading partner in 2019, bilateral trade with China is important to the U.S. economy, and the recent sharp downturn in activity affects a wide range of U.S. industries. Total U.S. trade with the world (the sum of exports and imports of goods and services)
From Beijing to Change Narrative About COVID-19,” SupChina, March 23, 2020, https://supchina.com/2020/03/23/mask-diplomacy-from-beijing-to-change-narrative-about-covid-19/.
5 Norihiko Sirouzu and Yilei Sun, “As China’s ‘Detroit’ Reopens, World’s Automakers Worry About Disruptions,” Reuters, March 8, 2020.
Congressional Research Service
2
link to page 8 COVID-19: China Medical Supply Chains and Broader Trade Issues
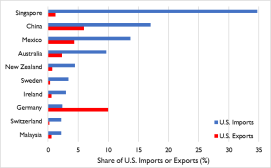
was $5.6 trillion in 2019, equivalent to 26% of U.S. gross domestic product (GDP); China accounts for 11% of U.S. trade.66 Key facts about the relationship include the following:7
China'7 China’s, total merchandise trade with the United States in 2019 amounted to $558.9 billion;-
China is the United States
'’ third largest export market for goods. U.S. goods exports to China in 2019 were valued at $106.6 billion in 2019; - China is the top source of U.S. imports. U.S. goods imports from China reached $452.2 billion in 2019;
- U.S. services exports to China in 2019 were valued at $56.7 billion (mostly travel and transport);
- U.S. services imports from China in 2019 were valued at $18 billion (about half of this amount was travel and transport); and
-
U.S. foreign direct investment (FDI) stock in China in 2018 reached $116.5
billion while China
'’s FDI stock in the United States reached $60.2 billion in 2018. -
Top U.S. exports to China include semiconductor chips, devices, parts and
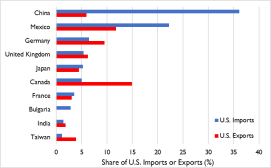
manufacturing machines; agriculture; aircraft, turbojets, turbo propellers, and gas turbines; optical and medical equipment; autos; plastics; and pharmaceutical products (Figure 1).
. - Top U.S. imports from China include microelectronics (computers and cell
phones) and appliances, furniture, bedding and lighting; toys, games and sports equipment; plastics; knitted and non-knitted apparel, textile fabric, linens, and footwear; auto parts; articles of iron and steel; medical and surgical instruments; and, organic chemicals (including active pharmaceutical ingredients and antibiotics).
6 CRS calculations based on data from the U.S. Department of Commerce, Bureau of Economic Analysis, “Gross Domestic Product, Fourth Quarter and Year 2019 (Second Estimate),” and “U.S. International Trade in Goods and Services, January 2020.” Total U.S.-China trade amounted to $635.3 billion in 2019.
7 The following data is sourced from the U.S. Department of Commerce, Bureau of Economic Analysis’ International Transactions.
Congressional Research Service
3
COVID-19: China Medical Supply Chains and Broader Trade Issues
Figure 1. U.S.-China Trade in 2019
antibiotics).
|
 |
Source: Congressional Research Service (CRS) with data from Global Trade Atlas.
|
China First Quarter (Q1) 2020 Slowdown Effects on U.S. Industries
Since Beginning in late January, the outbreak of COVID-19 in China has had a direct economic impact on U.S. firms that operate in China, export to or sell goods and services directly in China, or depend on Chinese goods and services for their operations in the United States and abroad. Some analysts estimate that China experienced a sharp drop in economic growth by as much as 9% in Q1 2020 and a 17.2% drop in exports in January-February 2020, compared to the same period in 2019.8 China'8
8 Ryan Woo, Se Young Lee, David Stanway, and Andrew Galbraith, “Goldman Sees China’s Economy Shrinking 9 Percent in First Quarter Amid Coronavirus Outbreak,” Reuters, March 16, 2020, https://www.reuters.com/article/us-health-coronavirus-china-toll/goldman-sees-chinas-economy-shrinking-9-in-first-quarter-amid-coronavirus-outbreak-idUSKBN21340T.
Congressional Research Service
4
COVID-19: China Medical Supply Chains and Broader Trade Issues
China’s economy is globally connected through trade, investment, and tourism. The economic slowdown and global spread of COVID-19, combined with global travel and transportation restrictions and other effects, are now causingcaused worldwide economic fallout. Indicators in key industries, include:
China has China recorded a sharp downturn in microelectronics production and sales and the United States could experience a similar drop due to a potential gap in availability. Almost half the value of U.S. imports from China in 2019 was-
Foxconn, a Taiwan firm that produces the iPhone for Apple in China, received
formal government permission to reopen its facilities in mid-February, but
hasfaced challenges because of quarantine and transportation restrictions. Foxconn'’s plan to offer $1,000 to each returning worker suggests potential lingering concerns about the risk of infection or other labor constraints. The companymay also facealso may have faced supply constraints of key microelectronics inputs.99 Other companies that use Foxconn for contract manufacturing in China include Amazon, Cisco, Dell, Google, Hewlett Packard, Nintendo, and Sony, as well as Chinese firms Huawei and Xiaomi.10 - 10
The U.S. auto industry and manufacturers in South Korea, Japan, and Germany
quickly faced manufacturing bottlenecks because of the lack of availability of auto parts supplies from China. The spread of COVID-19 to other major auto manufacturing markets, including the United States, Germany, Japan and South Korea
may poseimposed additional constraints on auto manufacturing and sales. China exported $9.6 billion in autopartsparts to the United States in 2019. - .
U.S. manufacturing
facesfaced potential shortages of intermediate inputs for steelmaking and heavy manufacturing, such as refined manganese metal, ferrosilicon, and ferrovanadium. Manganese and ferrovanadium are steel strengtheners that depend on China-based processing. While manganese is mined around the world, China controls 97% of manganese processing. Ferrosilicon is used to extract oxygen from liquid steel, and is mostly produced in China.1111 China exportedalmostalmost $10 billion in iron and steel products to the United States in2019. - 2019.
U.S. retailers, tourism, and service providers that rely on the Chinese consumer
base have also taken a hit in China. Many closed or significantly curtailed operations. U.S. retailers reduced operating hours or shuttered stores in response to COVID-19.
1212 For example, Starbucks closed about half its 4,200 retail outlets in China between late January and late February.1313 Retailers and tourism service providers around the world have seen significantly reduced revenue as fewer 9 “Apple Supplier Foxconn Expects Coronavirus-Hit Labor Shortage in China to Ease,” The Wall Street Journal, March 3, 2020. 10 Duncan Riley, “Apple and Others May Have Avoided Supply Shortages as some Foxconn Plants Reopen in China,” Silicon Angle, February 11, 2020. 11 Alistair MacDonald, “Steelmakers Rely Heavily on China,” The Wall Street Journal, March 6, 2020. 12 Samantha McDonald, “Columbia, Burberry and 12 Other Fashion Firms Are Closing Stores Due to Coronavirus,” Footwear News, February 27, 2020. 13 Hayley Peterson, “Starbucks Reopens Most Stores in China, Citing ‘Early Signs of Recovery,’ From Coronavirus,” Business Insider, February 27, 2020. Congressional Research Service 5 COVID-19: China Medical Supply Chains and Broader Trade Issuesproviders around the world have seen significantly reduced revenue as fewerChinese citizens travel abroadChina'China’s outbound tourism spending in 2018 was $277 billion, of which an estimated $36 billion was in the United States.14
14 Transportation, Logistics and Broader Considerations
Measures to contain the COVID-19 outbreak have significantly curtailed global transportation links. The consequence is the prevention of, preventing the transport of many products and manufacturing inputs. Passenger air traffic has slowed significantly, taking offline significant air cargo capacity for microelectronics and other products that ship by air. Container shipments are also constrained by the current backlog and dependence on domestic trucking and rail transportation, as well as on the ability of countries to staff port operations.
U.S. airlines started suspending flights to China in late January 2020 and have suspended other routes as COVID-19 has spread globally. United Airlines announced steep flight cuts and said in early March 2020 that ticket bookings were down 70% for Asia-Pacific flights, noting that this downturn was magnified by a surge in flight cancellations. The company noted that revenue in April and May could drop as much as 70%.1515 While Federal Express (FedEx) and United Parcel Service (UPS) announced in early March that they continued to run flights in and out of affected countries, they warned that limitations on travel could delay some shipments, although freight carriers are now starting to repurpose passenger flights for cargo which could help expand capacity.1616 Quarantine of aircrew and restrictions on the ground in China with regard to labor, production, supply and logistics likely significantly curtailed shipments. On March 26, 2020, the Civil Aviation Administration of China (CAAC) restricted all airlines running passenger flights in and out of China to one flight per week, further constraining air freight capacity.17
Container shipping from China has faced serious constraints17 In September 2020, CAAC announced the resumption of direct flights to Beijing from eight countries including Cambodia, Canada, Denmark, Greece, Sweden, and Thailand. Additionally, domestic passenger volumes in China appear to have reached 90% of pre-pandemic levels.18 Further recovery in air travel could lead to increased air freight capacity for shipments to and from China.19
Container shipping from China faced serious logjams because of shortages of workers and trucking constraints. These constraints are affectinglogjams affected both U.S. imports to and exports from China. The Port of Los Angeles has announced that shipmentsannounced shipment cuts by 25% that were scheduled from China between February and April 2020February and April 2020 have been cut by 25%. Los Angeles and Long Beach ports project a 15% to 17% drop in cargo volumes in Q1 2020. One in nine Southern California jobs is tied to the ports, including people who work on the docks, drive trucks, and move boxes in warehouses, according to the Executive Director of the Port of Los Angeles.18 The20 In March, the Port Authority of New York and New Jersey has requested $1.9 billion in federal aid to offset a forecasted 30% year-on-year drop in cargo volumes.19
In the immediate term, shipping and logistical constraints are slowing
14 United Nations World Tourism Organization, “Exports from International Tourism Hit USD 1.7 Trillion,” June 6, 2019, https://www.unwto.org/global/press-release/2019-06-06/exports-international-tourism-hit-usd-17-trillion; U.S. International Trade Administration National Travel and Tourism Office, “Fast Facts: United States Travel and Tourism Industry 2018,” October 2019, https://travel.trade.gov/outreachpages/download_data_table/Fast_Facts_2018.pdf. 15 Dawn Gilbertson, “Coronavirus Travel Fallout: American, Delta Cutting Flights as Demand Sinks, Joining United and Other,” USA Today, March 10, 2020. 16 “FedEx, UPS Warn of Delivery Delays, JPMorgan Tests Virus Contingency Plan,” PYMNTS.com, March 3, 2020. 17 Ministry of Foreign Affairs of the People’s Republic of China, “CAAC to Further Reduce International Passenger Flights,” press release, March 27, 2020, https://www.fmprc.gov.cn/mfa_eng/topics_665678/kjgzbdfyyq/t1762623.shtml.
18 Reuters, “Cheap seats give Chinese airlines a much-needed bounce,” September 15, 2020. 19 Reuters, “China will gradually resume direct international flights to Beijing,” September 2, 2020. 20 Margot Roosevelt, “Truckers, Dockworkers Suffer as Coronavirus Chokes L.A., Long Beach Ports Cargo, Los Angeles Times, March 7, 2020.
Congressional Research Service
6
COVID-19: China Medical Supply Chains and Broader Trade Issues
cargo volumes, and in July requested $3 billion to offset revenue losses stemming from a sharp decline in passenger volumes.21
In the immediate term, shipping and logistical constraints slowed U.S. exports to Asia. U.S. exporters of meat, poultry, hay, oranges and other produce are reportingreported in March 2020 that refrigerated containers are in short supply and cold storage facilities arewere overflowing with inventory.2022 U.S. and global manufacturing—including production that recently shifted out of China to other parts of Asia and to Mexico—is still recoveringtook time to recover from disruptions in Chinese supply. Vietnam, Taiwan, Malaysia, South Korea, Japan, Thailand, and Singapore all have strong supply chain links with China and reported Q1 supply shortages.21
23
Even as China'’s production resumesresumed, these Asian countries are now grapplinggrappled with their own COVID-19 outbreaks, further complicating recovery. The situation iswas exacerbated by spread of COVID-19 in other important manufacturing markets such as South Korea, Italy, Germany, and Mexico. Disruptions in Chinese supply chains were initially expected to have a limited macroeconomic effect on developed markets in the short term, but as the outbreak has spread globally and Chinese firms and logistics operations have struggled to return to full capacity, a wide range of U.S. imports from China, including raw materials, intermediate industrial inputs, and consumer products, are likely to be in short supply faced severe supply constraints. U.S. firms with operations in China or that depend on production in China have begun to consider diversification away from China and may face further pressure to establishChina may be prompted to diversify away from China and begin establishing new supply chains. The head of the EU Chamber of Commerce in China said in late February 2020 that the disruption from COVID-19 had driven home the need for foreign companies to diversify away from China.22
24 In April 2020, the Japanese government earmarked $2.2 billion of a broader economic stimulus package to help companies shift production out of China and to production sites in either Japan or Southeast Asia. In July, Japan’s Ministry of Economy, Trade, and Industry announced that 87 firms had agreed to shift production out of China and would receive funding, and in September 2020, added India and Bangladesh to its list of eligible alternative production sites.25 In August 2020, Australia, Japan, and India, announced a collaboration to incentivize companies to diversify supply chains from China for economic and geopolitical reasons.26
21 Port Authority of New York and New Jersey, “Port Authority Urgently Calls for Congress to Act on Request for $3 Billion in Federal Relief Following Precipitous Decline in Passenger Volumes Caused by COVID-19 Pandemic,” press release, July 29, 2020; Lee Hong Liang, “Port Authority of New York and New Jersey seeks $1.9bn bailout amid COVID-19,” Seatrade Maritime News, March 25, 2020; Port Authority of New York and New Jersey, Letter to Members of the New York and New Jersey Congressional Delegations, letter, March 19, 2020, https://www.politico.com/states/f/?id=00000170-fadd-d9d1-a3f3-fbdd91ed0000.
22 Jacob Bunge, “Meat Stockpiles Surge as Coronavirus Epidemic Curbs Exports,” The Wall Street Journal, March 2, 2020.
23 Trinh Nguyen, “The Economic Fallout of the Coronavirus in Southeast Asia,” Carnegie Endowment for International Peace, February 13, 2020.
24 “China virus outbreak threatens global drug supplies: European business group,” Reuters, February 17, 2020. 25 Isabel Reynolds and Emi Urabe, “Japan to Fund Firms to Shift Production Out of China,” Bloomberg, April 8, 2020; “Japan reveals 87 projects eligible for ‘China exit ‘subsidies,” Nikkei Asian Review, July 17, 2020. 26 Ministry of Economy, Trade, and Industry of the Government of Japan, “Australia-India-Japan Economic Ministers’ Joint Statement on Supply Chain Resilience,” September 1, 2020, https://www.meti.go.jp/press/2020/09/20200901008/20200901008-1.pdf; Kiran Sharma, “Japan, India, and Australia aim to steer supply chains around China,” Nikkei Asian Review, September 1, 2020.
Congressional Research Service
7
COVID-19: China Medical Supply Chains and Broader Trade Issues
Prospects for U.S. Exports Prospects for U.S. Exports
Within this context, U.S. firms may find some opportunities to increase exports to China, so long as global port operations resume and current logjams are resolved. Increased U.S. exports could be driven in part by recent tariff liberalization’ resumption of exports to China have depended on the resumption of global port operations and China’s economic recovery. While U.S. exports potentially would benefit from recent tariff liberalization, U.S. exports to China have been slow and uneven in their recovery. As part of the phase one trade deal that the United States and China signed in mid-January 2020 to resolve some issues the United States raised under Section 301, the United States and China agreed, effective February 14, 2020, to cut by 50% the tariffs they imposed in September 2019. China announced a tariff exemption process for 700 tariff lines, including some agriculture, medical supplies, raw materials, and industrial inputs.
With China'’s recovery, the U.S. government could press China to make up for lost time on U.S. purchases. COVID-19 may makehas made it difficult for both sides to meet these targets, however, given the economic fallout in both countries. China’s efforts to diversify import sources for key goods—such as energy and agriculture—have potentially undercut China’s capacity to meet its U.S. commitments. China imported 53.18 million tons of crude oil and replenished its strategic petroleum reserves from non-U.S. sources during the March 2020 collapse in global oil prices.27 The sustained outbreak of African Swine Flu in China has fueled an uptick in China’s pork imports from the United States, but overall agricultural purchases remain below previous years and still fall short of negotiated targets. the economic fallout in both countries. As part of the phase one trade deal, China committed to purchase at least $200 billion above a 2017 baseline amount of U.S. agriculture ($32 billion), energy ($52.4 billion), manufacturing goods ($77.7 billion), and services ($37.9 billion) between January 1, 2020 and December 31, 2021.2328 Regarding agriculture, in November 2019, China's ’s National Development and Reform Commission (NDRC) announced detailed rules for the application and allocation of grain and cotton import tariff-rate quotas for 2020 that specify imports for wheat (9.636 million tons, 90% state-owned trade), corn (7.2 million tons, 60% state-owned trade), rice (5.32 million tons, 50% state trade), and cotton (894,000 tons, 33% state-owned trade).2429 NDRC included in these rules a requirement that companies applying for tariff-rate quotas must have a "“positive record"” in China'’s corporate social credit system.2530 This requirement allows the Chinese government to restrict or impose terms on certain U.S. cotton exporters. China could use this requirement to create counter pressure in response to recent U.S. congressional action to block U.S. imports of textiles and apparel that contain cotton from China'China’s Xinjiang region due to concerns over forced labor there. 26 31 With falling oil prices, China would arguably have to buy a significant larger volume of goods to reach its purchase obligations that are benchmarked by dollar value.
27 Clyde Russell, “Column: China’s record crude oil, copper imports are more history lesson than predictor,” Reuters, July 14, 2020.
28 Office of the United States Trade Representative, “Economic and Trade Agreement Between the Government of the United States of America and the Government of the People’s Republic of China,” January 15, 2020.
29 National Development and Reform Commission of the People’s Republic of China, “关于 2020 年粮食棉花进口关税配额申请和分配细则的公告 2019 年第 9 号 (Announcement Regarding Application and Distribution Rules for Import Tariff Rate Quotas for Grain and Cotton)” September 29, 2019, https://www.ndrc.gov.cn/xxgk/zcfb/gg/201909/t20190930_1181887.html. 30 CRS In Focus IF11342, China’s Corporate Social Credit System, by Michael D. Sutherland. 31 Austin Ramzy, “U.S. Lawmakers Propose Tough Limits on Imports from Xinjiang,” The New York Times, March 11, 2020.
Congressional Research Service
8
link to page 56 COVID-19: China Medical Supply Chains and Broader Trade Issues
Force Majeure Provisions
The crisis also calledForce Majeure Provisions
The crisis is also calling into question China'’s ability to implement the U.S.-China phase one trade deal signed in January 2020. The agreement has a force majeure provision—which allows parties to opt out of contractual obligations without legal penalty because of developments beyond their control—that could give China flexibility in implementing its commitments.2732 The deal was finalized in December 2019 and signed in mid-January 2020, when Chinese officials reportedly knew about the severity of the COVID-19 outbreak in Wuhan, which raises questions about the rationale and timing of the decision to include the force majeure provision. A factor further complicating the potential for resumption and expansion of U.S. exports is Chinese companies' ’ invocation of force majeure certifications. For example, China National Petroleum Company (CNPC) used the outbreak of COVID-19 to declare force majeure in cancelling some liquefied natural gas (LNG) imports, a move followed by a downturn in overall oil and gas demand. The Ministry of Commerce has since provided free certifications to Chinese companies that need to declare force majeure.2833 Chinese companies and courts rely on an interpretation of force majeure that is different from the standard legal interpretation in the United States, which allows both parties to cancel contract terms and revert to a pre-contract baseline. In China, force majeure is used to cancel an obligation by the party invoking the provision while the other party may still be obligated to perform and honor contract terms. For example, if a payment is blocked or forgiven by the Chinese government, the other party may still be expected to perform according to the contract terms without the foreign party being reimbursed for any additional costs incurred. Moreover, Chinese courts are unlikely to allow foreign firms to prosecute Chinese firms that do not perform according to their contracts.29
34
U.S. Reliance on China for Health Care and Medical Products
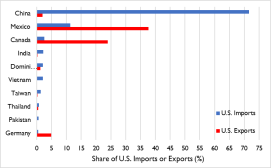
Products In the midst of the pandemic, Congress is expressing a strong interest in responding to U.S. shortages of medical supplies—including PPE and pharmaceuticals—as the United States steps up efforts to contain and counter COVID-19 with limited domestic stockpiles and constraints on U.S. industrial capacity. Because of China'’s role as a major U.S. and global supplier of medical PPE, medical devices, antibiotics, and active pharmaceutical ingredients (Appendix B)A), reduced exports from China have led to shortages of critical medical supplies in the United States.3035 While some analysts and industry groups have pointed to tariffs as a disincentive to U.S. imports of health and medical products, supply shortages due to the sharp spike in demand, as well as the nationalization and diversion of supply to China, appear to be stronger drivers. According to China Customs data, in 2019 China exported $9.8 billion in medical supplies and $7.4 billion in
32 Office of the United States Trade Representative, “Economic and Trade Agreement Between the Government of the United states of America and the Government of the People’s Republic of China,” January 15, 2020, Article 6.2. 33 Zhou Xin, “Coronavirus: Doubts Raised Over Whether Chinese Companies Can Use Force Majeure to Counter Risks,” South China Morning Post, February 25, 2020, https://www.scmp.com/economy/china-economy/article/3052277/coronavirus-doubts-raised-over-whether-chinese-companies-can.
34 Dan Harris, “Force Majeure in the Time of Coronavirus,” China Law Blog, Harris Bricken, February 27, 2020, https://www.chinalawblog.com/2020/02/force-majeure-in-the-time-of-coronavirus.html.
35 Finbarr Bermingham and Su-Lin Tan, “Coronavirus: China’s mas-making juggernaut cranks into gear, sparking fears of over-reliance on world’s workshop,” South China Morning Post, March 12, 2020, https://www.scmp.com/economy/global-economy/article/3074821/coronavirus-chinas-mask-making-juggernaut-cranks-gear; U.S. Food and Drug Administration, “Coronavirus (COVID-19) Supply Chain Update,” Press release, February 27, 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update.
Congressional Research Service
9
link to page 14 link to page 15
COVID-19: China Medical Supply Chains and Broader Trade Issues
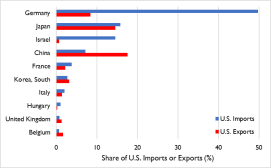
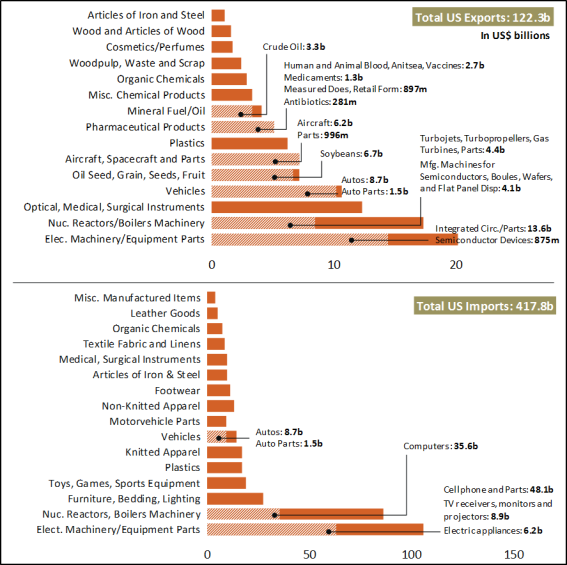
China Customs data, in 2019 China exported $9.8 billion in medical supplies and $7.4 billion in organic chemicals—a figure that includes active pharmaceutical ingredients and antibiotics—to the United States. While there are no internationally- agreed guidelines and standards for classifying these products, U.S. imports of pharmaceuticals, medical equipment and products, and related supplies are estimated to have been approximately $20.7 billion (or 9.2% of U.S. imports), according to CRS calculations using official U.S. data (Figure 2 and Table 1).
See Figure 2 and Table 1). This number likely understates the extent to which the United States relies on China for pharmaceuticals and medical equipment, products, and supplies. Some foreign products may contain Chinese inputs or components, which may or may not have been substantially transformed in other countries. However, they may not always be classified as Chinese in origin when imported into the United States. This is due, in part, to the “substantial transformation” test—used by U.S. Customs and Border Protection (CBP) to determine a product’s country of origin for trade purposes—which some consider to be complex, fact-specific, and subject to interpretation on a case-by-case basis that can be inconsistent and subjective.36 Additionally, there have been reported cases of Chinese-origin products being declared as non-Chinese in origin upon their importation into the United States (e.g., firms in other countries importing products from China and relabeling them for export to the United States to avoid tariffs).37 This number also likely understates U.S. API imports from China because U.S. direct and indirect imports of API from China may not be classified for such manufacturing use when imported into the United States.
Figure 2. U.S. Imports of Pharmaceuticals and Medical Equipment, Products, and
|
 |
Supplies in 2019
Source: CRS using the World Customs Organization Notes: The shares presented here cover product categories at the HTS six-digit level. |
China’s 9.2% share of U.S. imports likely understates the extent to which the United States relies on China for pharmaceuticals and medical equipment, products, and supplies because of how these imports are classified. Table 1. Select U.S. Imports from China in 2019
Value (U.S. Dollars) and Share of U.S. Imports (%)
Share of
U.S.
HTS
Value
Imports
Number
Description
(US$)
(%)
30
Pharmaceutical Products
1,560,469,274
1.2
3005.90
Medical Wadding, Gauze, Bandages, and Similar Articles
314,187,928
49.8
3001.90
Heparin and Its Salts
189,703,230
43.1
3005.10
Adhesive Dressing Articles
179,153,921
28.8
3006.50
First-Aid Boxes and Kits
27,482,506
72.4
3006.70
Gel Preparations (Lubricants) for Operations or
7,487,524
20.8
Physical Exams
3002.11
Malaria Diagnostic Test Kits
914,555
57.7
2941
Antibiotics
307,137,836
35.9
2941.30
Tetracyclines and Derivatives
93,302,575
90.1
2941.10
Penicil in and Derivatives
59,093,397
51.8
2941.50
Erythromycin and Derivatives
4,659,438
23.5
2941.20
Streptomycins and Derivatives
4,453,931
30.1
2941.40
Chloramphenicol and Derivatives
921,074
93.2
2941.90
Other Antibiotics (NESOI)
144,707,421
24.0
9018
Medical Instruments, Appliances, and Parts (Including
1,700,501,270
6.2
Electro-Medical and Sight-Testing)
9018.19
Electro-Diagnostic Equipment and other Apparatus For
368,723,243
9.7
Value (U.S. Dollars) and Share of U.S. Imports (%)
|
HTS Number |
Description |
Value (US$) |
Share of U.S. Imports (%) |
|
30 |
Pharmaceutical Products |
1,560,469,274 |
1.2 |
|
3005.90 |
Medical Wadding, Gauze, Bandages, and Similar Articles |
314,187,928 |
49.8 |
|
3001.90 |
Heparin and Its Salts |
189,703,230 |
43.1 |
|
3005.10 |
Adhesive Dressing Articles |
179,153,921 |
28.8 |
|
3006.50 |
First-Aid Boxes and Kits |
27,482,506 |
72.4 |
|
3006.70 |
Gel Preparations (Lubricants) for Operations or Physical Exams |
7,487,524 |
20.8 |
|
3002.11 |
Malaria Diagnostic Test Kits |
914,555 |
57.7 |
|
2941 |
Antibiotics |
307,137,836 |
35.9 |
|
2941.30 |
Tetracyclines and Derivatives |
93,302,575 |
90.1 |
|
2941.10 |
Penicillin and Derivatives |
59,093,397 |
51.8 |
|
2941.50 |
Erythromycin and Derivatives |
4,659,438 |
23.5 |
|
2941.20 |
Streptomycins and Derivatives |
4,453,931 |
30.1 |
|
2941.40 |
Chloramphenicol and Derivatives |
921,074 |
93.2 |
|
2941.90 |
Other Antibiotics (NESOI) |
144,707,421 |
24.0 |
|
9018 |
Medical Instruments, Appliances, and Parts (Including Electro-Medical and Sight-Testing) |
1,700,501,270 |
6.2 |
|
9018.19 |
|
368,723,243 |
9.7 |
|
9018.31 |
Syringes (Including Parts and Accessories) |
106,902,008 |
14.4 |
|
9018.12 |
Ultrasonic Scanning Apparatus |
78,806,780 |
19.9 |
|
9018.20 |
Ultraviolet or Infrared Ray Apparatus (Including Parts and Accessories) |
11,493,518 |
14.6 |
|
9019 |
Mechano-Therapy and Respiration Apparatus, Including Parts and Accessories |
1,386,955,875 |
32.5 |
|
9019.10.20 |
Mechano-Therapy Appliances and Massage Apparatus |
918,922,381 |
58.5 |
|
9019.20.00 |
Medical Ventilators and Other Artificial Respiration Equipment |
449,688,296 |
17.0 |
|
9019.10.60 |
Select Psychological Aptitude Testing Equipment |
12,155,935 |
29.8 |
|
9019.10.40 |
Electrical Psychological Aptitude Testing Equipment |
6,189,263 |
57.9 |
|
9020 |
|
10,002,578 |
4.0 |
|
9020.00.60 |
Breathing Appliances and Gas Masks |
5,448,928 |
3.1 |
|
9020.00.90 |
Parts and Accessories of Breathing Appliances and Gas Masks |
4,124,104 |
7.2 |
|
9021 |
Orthopedic and Other Appliances to Compensate for a Defect, Including Parts and Accessories |
930,437,769 |
6.8 |
|
9021.10 |
Orthopedic of Fracture Appliances |
323,279,299 |
13.1 |
|
9022 |
X-Ray Apparatus and Parts |
492,398,140 |
11.0 |
|
9022.12 |
Computed Tomography (CT) Apparatus |
49,051,037 |
7.2 |
Source: CRS with data from the U.S. International Trade Commission's DataWeb.
China Nationalizes Medical Production and Supply
China Nationalizes Medical Production and Supply In early February 2020, the Chinese government nationalized control of the production and dissemination of medical supplies in China. Concerned about shortages and its ability to contain the COVID-19, the Chinese government transferred authority over the production and distribution of medical supplies from the Ministry of Information Industry and Technology (MIIT) to the NDRC, China'China’s powerful central economic planning ministry. NDRC commandeered medical manufacturing and logistics down to the factory level and has been directing the production and distribution of all medical-related production, including U.S. companies'’ production lines in China, for domestic use.3138 In response to government directives, foreign firms with significant production capacity in China, including 3M, Foxconn, and General Motors, shifted significant elements of their operations to manufacturing medical PPE.3239 By late February 2020, China had 38 Zhang Pinghui and Zhou Xin, “Coronavirus: China Shifts Responsibility Over Medical Supplies Amid Mask Shortage, Rising Death Toll,” South China Morning Post, February 3, 2020, updated on February 14, 2020, https://www.scmp.com/economy/china-economy/article/3048744/coronavirus-mask-shortage-prompts-beijing-tweak-authority. 39 “Exclusive: Unilever, 3M, on list of firms eligible for China loans to ease coronavirus crisis – sources,” Reuters,
Congressional Research Service
13
link to page 19 link to page 20 link to page 19 COVID-19: China Medical Supply Chains and Broader Trade Issues
By late February 2020, China had ramped up face mask production—both basic surgical masks and N95 masks—from a baseline of 20 million a day to over 100 million a day.
China'
China’s nationalization efforts, while understandable as part of its efforts to address an internal health crisis, may have denied the United States and other countries that depend on open and free markets for their health care supply chains timely access to critical medical supplies (See Table 2 and Table 3).33.40 On February 3, 2020, China'’s Ministry of Commerce directed its bureaucracy, local governments and industry to secure critical technology, medical supplies, and medical-related raw material inputs from the global market,3441 a situation that likely further exacerbated supply shortages in the United States and other markets. To ensure sufficient domestic supplies to counter COVID-19, China'’s Ministry of Commerce (MOFCOM) also called on its regional offices in China and overseas to work with PRC industry associations to prioritize securing supplies from global sources and importing these products. The Ministry of Commerce provided a list of 51 medical suppliers and distributors in 14 countries and regions to target in quickly assuring supply. The Ministry also prioritized food security and the need to increase meat imports.3542 China'’s trade data shows that these policies led to steep increases in China'’s imports of essential PPE and medical supplies, including the raw materials needed to make products such as N95 masks. The policies also contributed to sharp decreases in China'’s exports of these critical medical products to the world. (See Table 2.)
On March 29, 2020, the Australian government imposed new temporary restrictions on all foreign investment proposals in Australia out of concern that strategic investors—particularly those of Chinese origin—might target distressed assets. This comes after authorities discovered two instances of Chinese property developers in Australia purchasing large volumes of medical supplies (and precious metals) for shipment to China.3643 Risland—a wholly- owned subsidiary of one of China'’s largest property developers, Country Garden Holdings—reportedly shipped 82 tons of medical supplies from Australia to China on February 24, 2020. The shipment included February 13, 2020, https://www.reuters.com/article/us-china-health-lending-exclusive-idUSKBN20D0SQ; Yang Jian, “GM, Wuling venture begins output of machines to make face masks,” Automotive News China, February 20, 2020, https://www.autonews.com/china/gm-wuling-venture-begins-output-machines-make-face-masks.
40 Zhang Pinghui and Zhou Xin, “Coronavirus: China Shifts Responsibility Over Medical Supplies Amid Mask Shortage, Rising Death Toll,” South China Morning Post, February 3, 2020, updated on February 14, 2020, https://www.scmp.com/economy/china-economy/article/3048744/coronavirus-mask-shortage-prompts-beijing-tweak-authority; Finbarr Bermingham and Su-Lin Tan, “Coronavirus: China’s Mask Making Juggernaut Cranks Into Gear, Sparking Fears of Overreliance on World’s Workshop,” South China Morning Post, March 12, 2020, https://www.scmp.com/economy/global-economy/article/3074821/coronavirus-chinas-mask-making-juggernaut-cranks-gear; Engen Tham, Cheng Leng, and Zhang Yan, “Exclusive: Unilever, 3M on List of Firms Eligible for China Loans to Ease Coronavirus Crisis—Sources,” Reuters, February 19, 2020, https://www.reuters.com/article/us-china-health-lending-exclusive-idUSKBN20D0SQ; Yang Jian, “GM, Wuling Venture Begins Output of Machines to Make Face Masks, Automotive News, February 20, 2020, https://www.autonews.com/china/gm-wuling-venture-begins-output-machines-make-face-masks; and Luffy Liu, “700 Tech Companies in China Have Begun Making Masks,” EE Times, February 13, 2020, https://www.eetimes.com/700-tech-companies-in-china-have-begun-making-masks/. 41 Ministry of Commerce of the People’s Republic of China, “Circular on Further Facilitating the Import and Export of Technology During the Period of Epidemic Prevention and Control,” February 4, 2020, http://english.mofcom.gov.cn/article/newsrelease/significantnews/202002/20200202934774.shtml; and Ministry of Commerce of the People’s Republic of China, “Circular on Actively Expanding Imports to Combat Against Novel Coronavirus Epidemic,” February 6, 2020, http://english.mofcom.gov.cn/article/newsrelease/significantnews/202002/20200202934157.shtml.
42 Ministry of Commerce of the People’s Republic of China, “General Office of the Ministry of Commerce Issued the Circular on Actively Expanding Imports to Combat against Novel Coronavirus Epidemic,” press release, February 6, 2020.
43 Phillip Coorey, “China Spree Sparks FIRB Crackdown,” Financial Review, March 29, 2020, https://www.afr.com/politics/federal/china-spree-sparks-firb-crackdown-20200329-p54exo.
Congressional Research Service
14
COVID-19: China Medical Supply Chains and Broader Trade Issues
tons of medical supplies from Australia to China on February 24, 2020. The shipment included 100,000 medical gowns and 900,000 pairs of gloves.3744 Greenland Australia—a subsidiary of another large Chinese property developer backed by the Chinese government, Greenland Group—implemented instructions from the Chinese government to secure bulk supplies of medical items from the global market. Greenland reportedly sourced from Australia and other countries, 3 million protective masks, 700,000 hazmat suits, and 500,000 pairs of gloves for export to China over several weeks in January and February 2020.38
45
Table 2. Change in China'’s Exports and Imports of Select Medical Products
YTD 2019 (January-February) vs. YTD 2020 (January-February)
World
United States
Exports Imports Exports Imports
HS
%
%
%
%
Code
Description
Change
Change
Change
Change
6210.10
Garments, Made-Up of Fabrics of Felts and
-13
40,582
-21
297,288
YTD 2019 (January-February) vs. YTD 2020 (January-February)
|
HS Code |
Description |
World |
United States |
||
|
Exports % Change |
Imports % Change |
Exports % Change |
Imports % Change |
||
|
6210.10 |
|
-13 |
40,582 |
-21 |
297,288 |
|
6307.90* |
Made-Up Textile Articles* |
-16 |
2,176 |
-19 |
1,615 |
|
2939.80 |
Alkaloids |
13 |
1,019 |
-18 |
- |
|
4015.11 |
Surgical and Medical Gloves |
4 |
210 |
-8 |
93 |
|
3002.14 |
Immunological Products |
-30 |
197 |
121,302 |
626 |
|
3808.94 |
Disinfectants |
46 |
192 |
35 |
155 |
|
6210.30 |
Women's or Girls' Protective Garments |
-35 |
188 |
-48 |
-75 |
|
9004.90 |
Spectacles and Goggles |
-20 |
185 |
-12 |
164 |
|
9019.20 |
Medical Ventilators and Respiration Apparatus |
-20 |
174 |
-35 |
209 |
|
2936.26 |
Vitamin B12 and Its Derivatives |
-6 |
113 |
-21 |
-33 |
|
6506.10 |
Safety Headgear |
-15 |
106 |
-19 |
277 |
|
4015.19 |
Gloves |
-13 |
77 |
-35 |
514 |
|
8419.20 |
Medical, Surgical or Laboratory Sterilizers |
-34 |
66 |
-70 |
317 |
|
3926.20 |
Gloves, Mittens, and Mitts of Plastics |
-15 |
66 |
-13 |
74 |
|
9025.19 |
Thermometers and Pyrometers |
-12 |
65 |
-16 |
15 |
|
9020.00 |
Breathing Appliances and Gas Masks Having Mechanical Parts or Replaceable Filters |
-23 |
34 |
27 |
5 |
|
3005.90 |
Wadding, Gauze, Bandage, and Similar Articles for Medical, Surgical Purposes |
-12 |
27 |
-8 |
131 |
|
3004.20 |
Medicaments Containing Antibiotics |
-11 |
23 |
-18 |
69 |
|
4818.90 |
Bed Sheets and Similar Household or Hospital Articles of Paper |
-12 |
21 |
-7 |
-26 |
|
6505.00 |
Hats and Other Headgear of Textile Fabric |
-22 |
16 |
-24 |
-8 |
|
3702.10 |
X-Ray Film in Rolls |
-43 |
15 |
- |
50 |
|
9022.90 |
|
-11 |
15 |
-17 |
12 |
|
3402.20 |
Surface-Active Preparations, Washing Preparations, and Cleaning Preparations |
-18 |
8 |
-21 |
-18 |
|
9022.30 |
X-Ray Tubes |
-17 |
5 |
115 |
-6 |
|
6116.10 |
Gloves |
-16 |
-11 |
-21 |
-86 |
|
2936.27 |
Vitamin C |
-39 |
-16 |
-37 |
-95 |
|
9025.11 |
Thermometers and Pyrometers |
-31 |
-22 |
-10 |
299 |
|
6216.00 |
Gloves, Mittens, and Mitts |
-27 |
-22 |
-30 |
25 |
|
6210.40 |
Men's Or Boys' Protective Garments |
-25 |
-23 |
-28 |
-63 |
|
9018.31 |
Syringes |
-21 |
-28 |
-50 |
-18 |
|
2847.00 |
Hydrogen Peroxide |
-86 |
-29 |
-100 |
4 |
|
2941.10 |
Penicillin and Derivatives |
-24 |
-34 |
-17 |
- |
|
3004.42 |
Medicaments Containing Pseudoephedrine |
-61 |
- |
- |
- |
|
3003.60 |
Medicaments Containing Antimalarial Active Principles |
-98 |
- |
- |
- |
Source: CRS analysis with data from China Customs and Global Trade Atlas (March 31, 2020).
with data from China Customs and Global Trade Atlas (March 31, 2020). Notes: *N95 and other protective masks have historically been classified under tariff subheading 6307.99.9889, which includes other miscellaneous textile article made from similar materials.
Table 3. U.S. Imports from China in 2019: COVID-19 Related Medical Supplies
Value (U.S. Dollars), Share of U.S. Imports (%), and Change from Previous Year (%)
Share
of U.S.
Change
HS
China's
Imports
2019/18
Category
Product Description
Number
Rank
Value (US$)
(%)
(%)
I. COVID-19 Test
COVID-19 test kits (diagnostic
3822.00
7
212,319,127
5.4
2.7
Kits/Instruments
reagents based on polymerase chain
and Apparatus
reaction nucleic acid test)
Used in Diagnostic Tests
COVID-19 test kits (diagnostic
3002.15
16
21,754,253
0.1
-59.4
reagents based on immunological
reactions)
Congressional Research Service
16
COVID-19: China Medical Supply Chains and Broader Trade Issues
Share
of U.S.
Change
HS
China's
Imports
2019/18
Category
Product Description
Number
Rank
Value (US$)
(%)
(%)
COVID-19 diagnostic test
9027.80
3
155,359,874
9.5
-1.4
Value (U.S. Dollars), Share of U.S. Imports (%), and Change from Previous Year (%)
|
Category |
Product Description |
HS Number |
China's Rank |
Value (US$) |
Share of U.S. Imports (%) |
Change 2019/18 (%) |
|
I. COVID-19 Test Kits/Instruments and Apparatus Used in Diagnostic Tests |
COVID-19 test kits (diagnostic reagents based on polymerase chain reaction nucleic acid test) |
3822.00 |
7 |
212,319,127 |
5.4 |
2.7 |
|
COVID-19 test kits (diagnostic reagents based on immunological reactions) |
3002.15 |
16 |
21,754,253 |
0.1 |
-59.4 |
|
|
9027.80 |
3 |
155,359,874 |
9.5 |
-1.4 |
|
|
II. Protective Garments |
Face and Eye Protection |
|||||
|
6307.90 |
1 |
3,171,956,472 |
71.7 |
6.5 |
|
|
9020.00 |
7 |
10,002,578 |
4.0 |
26.3 |
|
|
Protective spectacles and goggles |
9004.90 |
1 |
503,787,243 |
54.8 |
6.8 |
|
|
Gloves |
||||||
|
Plastic gloves |
3926.20 |
1 |
863,056,388 |
77.2 |
-24.3 |
|
|
Surgical rubber gloves |
4015.11 |
6 |
1,081,073 |
0.3 |
9.6 |
|
|
Other rubber gloves |
4015.19 |
3 |
252,443,610 |
11.0 |
-5.6 |
|
|
Knitted or crocheted gloves which have been impregnated or covered with plastics or rubber |
6116.10 |
1 |
363,733,689 |
53.6 |
11.2 |
|
|
Textile gloves that are not knitted or crocheted |
6216.00 |
1 |
195,084,793 |
54.7 |
1.9 |
|
|
Other |
||||||
|
Disposable hair nets |
6505.00 |
1 |
934,958,363 |
51.9 |
-21.3 |
|
|
Protective garments for surgical/medical use made up of felt or nonwovens |
6210.10 |
1 |
440,561,626 |
54.3 |
-0.7 |
|
|
Other protective garments of textiles of rubberized textile fabrics or woven fabrics |
6210.20 |
1 |
27,688,815 |
64.2 |
15.4 |
|
|
6210.30 |
1 |
55,082,976 |
55.5 |
37.3 |
|
|
6210.40 |
1 |
323,357,757 |
44.8 |
5.0 |
|
|
6210.50 |
1 |
202,474,607 |
45.4 |
8.8 |
|
|
Liquid filled thermometer for direct reading |
9025.11 |
1 |
15,364,796 |
81.0 |
20.6 |
|
Other thermometers |
9025.19 |
1 |
217,189,968 |
36.2 |
-25.5 |
|
|
IV. Disinfectants and Sterilization Products |
Alcohol solution |
2207.10 |
23 |
25,420 |
0.0 |
154.2 |
|
Alcohol solution |
2208.90 |
2 |
40,916,542 |
2.1 |
-20.5 |
|
|
Hand sanitizer |
3824.99 |
2 |
365,615,644 |
15.3 |
-16.7 |
|
|
Hand sanitizer |
3402.20 |
3 |
39,757,125 |
7.6 |
-33.6 |
|
|
Medical, surgical or laboratory sterilizers |
8419.20 |
13 |
1,854,603 |
1.0 |
22.7 |
|
|
Hydrogen peroxide |
2847.00 |
15 |
10,089 |
0.0 |
-61.6 |
|
|
Hydrogen peroxide presented as a medicament |
3004.90 |
15 |
475,162,117 |
0.8 |
19.5 |
|
|
Other chemical disinfectants |
3808.94 |
2 |
8,841,055 |
10.3 |
132.5 |
|
|
V. Other Medical Devices |
Computed tomography (CT) scanners |
9022.12 |
4 |
49,051,037 |
7.2 |
-54.8 |
|
Extracorporeal membrane oxygenation |
9018.90 |
5 |
758,088,695 |
5.8 |
14.6 |
|
|
Medical ventilators and other oxygen therapy apparatus |
9019.20 |
2 |
449,688,296 |
17.0 |
-2.9 |
|
|
Patient monitoring devices |
9018.19 |
4 |
368,723,243 |
9.7 |
-10.1 |
|
|
VI. Medical Consumables |
Wadding, gauze, bandages, cotton sticks and similar articles |
3005.90 |
1 |
314,187,928 |
49.8 |
10.6 |
|
Syringes, with or without needles |
9018.31 |
2 |
106,902,008 |
14.4 |
6.3 |
|
|
Tubular metal needles and needles for sutures |
9018.32 |
8 |
22,465,545 |
2.9 |
11.6 |
|
|
Needles, catheters, cannulae, and the like |
9018.39 |
6 |
229,655,282 |
3.7 |
7.0 |
|
|
Intubation kits |
9018.90 |
5 |
758,088,695 |
5.8 |
14.6 |
|
|
Paper bed sheets |
4818.90 |
1 |
356,642,980 |
74.9 |
9.9 |
Source: CRS using the World Customs Organization's "HS Classification Reference for COVID-19 Medical Supplies" and data from Global Trade Atlas.
data from Global Trade Atlas. Notes: The international Harmonized System (HS) for classifying goods is a six-digit code system, and classification at the eight and ten-digit levels varies by country. The figures presented here may overestimate the actual value of U.S. imports of medical products, as it is not always possible to identify specific, individual products even at the most disaggregated level.
Implications of China'’s Export Constraints: U.S. Shortages and Policy Response
As the United States rampsramped up efforts to contain the spread of COVID-19, reduced production and exports of pharmaceuticals and PPE from China are exacerbatingexacerbated shortages of critical medical supplies. Minnesota-based 3M, a large-scale manufacturer of N95 respirators, for example, told The New York Times that all masks manufactured at its Shanghai factory were sold to meet China'China’s domestic demand; other mask manufacturers, such as Canada'’s Medicom, have stated that the Chinese government has not yet authorized them to export PPE.3946 China'’s Ministry of Commerce
46 Keith Bradsher and Liz Alderman, “The World Needs Masks. China Makes Them—But Has Been Hoarding Them,”
Congressional Research Service
18
COVID-19: China Medical Supply Chains and Broader Trade Issues
s Ministry of Commerce has claimed it is not imposing export restrictions on medical supplies,4047 but this statement may not apply to the current situation as all of China'’s domestic production is controlled by the government and geared toward domestic consumption.
U.S. national and state-level health authorities have been
Subsequently, China’s imposition of new export quality checks for PPE, particularly masks—implemented by China’s National Medical Products Administration (NMPA)—further slowed exports. On March 30, 2020, China’s Ministry of Commerce (MOFCOM) announced new qualifications for medical supply exports.48 It announced that all medical supplies related to COVID-19 would need to obtain qualifications from China’s National Medical Products Administration (NMPA). These new requirements appear aimed at addressing faulty PPE that several foreign government buyers found in large PPE shipments from China. By requiring new certification processes, the measures also appear to have slowed legitimate exports. Even companies producing in China for export that already had FDA approval had to meet these new PRC requirements.49 Related to MOFCOM’s announcement, on March 30, 2020, NMPA issued Regulatory Requirements and Standards for Coronavirus Testing Reagents and Protective Products. These new requirements regulated COVID-19 testing reagents as Class III medical equipment (highest risk level) and regulated medical-use masks and protective gear as Class II medical equipment, requiring producers to obtain licenses from provincial-level regulators prior to production. The NMPA classified regular masks and protective goggles as Class I medical equipment, requiring producers to file with local authorities. The NMPA also released registration information for seven COVID-19 products (testing kits, ventilators, medical protective wears, medical protective masks, medical surgical masks, single-use masks, and infrared thermometers-detectors).50
In addition to these new registration and inspection requirements, some U.S. legal experts observed that China may have used informal measures, such as administrative guidance, to prioritize exports to certain countries ahead of the United States.51 This may have been politically motivated, as China orchestrated publicized PPE deliveries to countries such as Serbia and Italy, which have signed on to China’s One Belt, One Road Initiative and whom China sees as important partners for investment and trade initiatives in Europe.52 China organized a range of government-to-government medical supply agreements around the world that sought to emphasize the importance of relations with China and the government’s goodwill, but ran into problems with many governments due to faulty PPE. In April and May 2020, Canada’s public health authority reported extensive problems with KN95 respirator masks from China that were counterfeit or otherwise failed to meet federal COVID-19 standards for medical use.53 Several
The New York Times, March 16, 2020, https://www.nytimes.com/2020/03/13/business/masks-china-coronavirus.html.
47 “China Imposes No Export Ban on Masks: Commerce Official,” Xinhua, March 5, 2020, http://english.www.gov.cn/statecouncil/ministries/202003/05/content_WS5e60c25dc6d0c201c2cbda0b.html.
48 State Council of the People’s Republic of China, “National Medical Product Administration Announcement No. 5 on the Orderly Export of Medical Products (国家药品监督管理局公告 2020 年第 5 号 关于有序开展医疗物资出口的公告), March 31, 2020, http://www.gov.cn/zhengce/zhengceku/2020-04/01/content_5497878.htm.
49 Kate O’Keefe, Liza Lin, and Eva Xiao, “China’s Export Restrictions Strand Medical Goods U.S. Needs to Fight Coronavirus, State Department Says,” The Wall Street Journal, April 16, 2020. 50 China’s National Medical Products Administration, http://www.nmpa.gov.cn/WS04/CL2590/complete. 51 “Navigating PPE Purchases from China,” Webinar with Harris Bricken Law Firm, April 23, 2020. 52 Eleanor Albert, “How a Pandemic Drew China and Serbia Closer,” The Diplomat, March 27, 2020; Xinhua, “Iron-clad China-Serbia friendship stronger in COVID-19 fight,” April 2, 2020; Reuters, “China sends medical supplies, experts to help Italy battle coronavirus,” March 13, 2020; Alicia Chen and Vanessa Molter, “Mask Diplomacy: Chinese Narratives in the COVID Era,” Stanford Freeman Spogli Institute for International Studies, June 16, 2020. 53 Jim Bronskill, “Federal Government Rejects 8 Million N95 Masks from a Single Distributor,” The Canadian Press,
Congressional Research Service
19
link to page 25
COVID-19: China Medical Supply Chains and Broader Trade Issues
European countries, including the Netherlands and Spain, reported faulty masks and COVID-19 test kits.54 Chinese propaganda efforts tied to the delivery of PPE were criticized in western media and by European Union officials as trying to capitalize on the crisis to try and divide Europe. Chinese media frequently conflated Chinese government-organized and publicized shipments of PPE that had been procured and paid for by foreign governments as aid.55
China’s exports of COVID-19-related PPE experienced a sharp uptick between March and June 2020, before tapering off in July with a particularly pronounced increase in N95 masks that peaked earlier than other products. China appears to have prioritized Europe in its export of N95 masks, a trend that is reflected both in overall increases and shifts in market share as a percentage of total imports. China’s market share as a percentage of EU imports of N95 masks rose by 16%, while its share of U.S. imports declined by 15%. China’s market share for N95 masks in Japan, Australia, and South Korea also declined somewhat. (See Table 4).
Figure 4. China’s Export of Select Covid-19-Related Products: Jan.-July 2020
Source: CRS with data from China Customs. Categories based on 6-digit product codes outlined by the World Customs Organization in “HS Classification Reference for Covid-19 Medical Supplies,” ed. 2, as updated April 30, 2020. Notes: Changes in the value of trade are determined by both the quantity of trade and the price of the good. Many of these products experienced an increase in both price and quantity, during the March-July 2020 period. In defining these product groups, CRS has attempted to capture the most common HS categorizations for these items, however, some trade of these items may have been exported under additional HS codes not reflected here, and, conversely, the product groups are not exclusive to Covid-19-related gear and may include other similar trade. EU-27 includes the 27 member states of the European Union, excluding the United Kingdom. U.A.E. is the United Arab Emirates.
May 8, 2020; Evan Dyer, “Canadian-Approved N95 Masks Targeted by Chinese Counterfeiters,” CBC News, May 14, 2020, and Stephen Chase, “Canada Says One Million Face Masks from China Failed to Meet Federal Standards,” The Globe and Mail, April 24, 2020.
54 Elena Sanchez Nicolas, “EU Fighting Faulty Medical Supplies,” EU Observer, April 2, 2020. 55 Charlie Campbell, “China’s ‘Mask Diplomacy’ is Faltering. But the U.S. Isn’t Doing Any Better,” Time, April 3, 2020, and EU HRVP Josep Borrell, “The Coronavirus Pandemic and the New World it is Creating,” Official Website of the European Union, March 24, 2020.
Congressional Research Service
20
link to page 25
COVID-19: China Medical Supply Chains and Broader Trade Issues
a. HS 630790 is a higher-level classification that includes N95 masks and the Chinese standard KN95 masks
among other types of “Made-Up Textile Articles [not classified elsewhere].”
b. Other Face and Eye PPE is defined as HS 392690, 481890, 900490, and 902000. c. Gowns and Other PPE are defined as HS 392620, 401590, 481850, 621010, 621040, 621050, and 650500. d. PPE Gloves are defined as HS 392620, 401511, 401519, 611610, and 621600. e. Covid-19 Test Kits and Instructions are defined as HS 300215, 382100, 382200, and 902780.
Figure 5. China’s Exports of Select Covid-19-Related Products:
Comparison of March-July 2019 and March-July 2020
Source: CRS with data from China Customs. Categories based on 6-digit product codes outlined by the World Customs Organization in “HS Classification Reference for Covid-19 Medical Supplies,” ed. 2, as updated April 30, 2020. Notes: Changes in the value of trade are determined by both the quantity of trade and the price of the good. Many of these products experienced an increase in both price and quantity, during the 2020 period. For definitions of the product categories and other caveats, see the notes of Table 4.
Table 4. Top Partners: Value of China’s Exports of Select Covid-19-Related Medical
Goods
Comparison of the March-July Period in 2019 and 2020
USD, Millions
% Change
Market Share (%)
Mar.-
Mar.-July Mar.-July
Mar.-July
July
Mar.-July
Top Partners
2019
2020
2019
2019
2019
N95 Respirator Masks and Other Made-Up Textiles (HS 630790)a
EU-27
$409.7
$13,439.4
3180.2%
18.3%
34.6%
United States
$917.0
$9,910.2
980.7%
40.9%
25.5%
Japan
$199.5
$2,925.5
1366.7%
8.9%
7.5%
United
$105.5
$2,555.6
2321.8%
4.7%
6.6%
Kingdom
Congressional Research Service
21
link to page 25 link to page 25 COVID-19: China Medical Supply Chains and Broader Trade Issues
USD, Millions
% Change
Market Share (%)
Mar.-
Mar.-July Mar.-July
Mar.-July
July
Mar.-July
Top Partners
2019
2020
2019
2019
2019
Canada
$60.8
$1,223.1
1912.3%
2.7%
3.2%
Singapore
$13.6
$636.7
4565.6%
0.6%
1.6%
Australia
$55.8
$628.4
1026.6%
2.5%
1.6%
Russia
$17.5
$615.1
3416.4%
0.8%
1.6%
South Korea
$65.3
$579.9
788.0%
2.9%
1.5%
Mexico
$19.3
$401.6
1983.5%
0.9%
1.0%
All Other
$379.1
$5,929.7
1464.1%
16.9%
15.3%
World
$2,243.1
$38,845.2
1631.8%
100.0%
100.0%
Other Face and Eye Personal Protection Equipment (excluding N95 masks)b
United States
$1,750.9
$2,121.9
21.2%
27.0%
26.0%
EU-27
$1,065.2
$1,215.0
14.1%
16.4%
14.9%
United
$248.9
$538.4
116.3%
3.8%
6.6%
Kingdom
Japan
$424.3
$525.6
23.9%
6.6%
6.4%
Australia
$147.4
$323.9
119.8%
2.3%
4.0%
Hong Kong
$415.8
$313.0
-24.7%
6.4%
3.8%
South Korea
$176.1
$264.3
50.1%
2.7%
3.2%
Singapore
$127.4
$251.3
97.3%
2.0%
3.1%
Vietnam
$172.6
$236.9
37.3%
2.7%
2.9%
Canada
$174.0
$214.6
23.3%
2.7%
2.6%
All Other
$1,775.3
$2,164.4
21.9%
27.4%
26.5%
World
$6,477.9
$8,169.3
26.1%
100.0%
100.0%
Gowns and Other Personal Protection Garmentsc
United States
$1,071.4
$2,955.3
175.8%
30.0%
27.4%
EU-27
$962.9
$2,773.6
188.0%
26.9%
25.7%
United
$168.3
$1,120.5
565.7%
4.7%
10.4%
Kingdom
Canada
$113.0
$593.9
425.6%
3.2%
5.5%
Japan
$215.9
$550.3
154.9%
6.0%
5.1%
Russia
$94.3
$511.5
442.7%
2.6%
4.7%
Australia
$79.1
$264.0
233.6%
2.2%
2.4%
Philippines
$17.2
$139.4
709.7%
0.5%
1.3%
U.A.E
$21.4
$124.9
482.9%
0.6%
1.2%
Saudi Arabia
$42.9
$107.8
151.2%
1.2%
1.0%
Congressional Research Service
22
link to page 25 link to page 25 COVID-19: China Medical Supply Chains and Broader Trade Issues
USD, Millions
% Change
Market Share (%)
Mar.-
Mar.-July Mar.-July
Mar.-July
July
Mar.-July
Top Partners
2019
2020
2019
2019
2019
All Other
$787.9
$1,657.7
110.4%
22.0%
15.4%
World
$3,574.5
$10,798.9
202.1%
100.0%
100.0%
Gloves: Personal Protection Equipmentd
United States
$626.1
$1,309.9
109.2%
32.3%
33.0%
EU-27
$407.4
$899.9
120.9%
21.0%
22.6%
United
$70.6
$410.2
481.4%
3.6%
10.3%
Kingdom
Japan
$161.4
$259.6
60.9%
8.3%
6.5%
Canada
$64.5
$183.0
183.9%
3.3%
4.6%
Russia
$40.8
$85.2
108.7%
2.1%
2.1%
Australia
$34.1
$72.6
112.7%
1.8%
1.8%
U.A.E.
$22.1
$67.7
206.2%
1.1%
1.7%
Brazil
$41.0
$45.5
10.8%
2.1%
1.1%
South Korea
$35.5
$43.6
22.9%
1.8%
1.1%
All Other
$432.6
$598.5
38.3%
22.3%
15.1%
World
$1,936.2
$3,975.6
105.3%
100.0%
100.0%
Covid-19 Test Kits and Instrumentse
EU-27
$131.9
$606.5
360.0%
23.2%
22.2%
United States
$130.2
$377.9
190.2%
22.9%
13.8%
Hong Kong
$70.4
$370.2
426.0%
12.4%
13.5%
Brazil
$7.7
$178.6
2231.2%
1.4%
6.5%
Indonesia
$8.9
$89.6
902.1%
1.6%
3.3%
Philippines
$4.4
$87.4
1889.4%
0.8%
3.2%
Russia
$4.9
$82.7
1578.8%
0.9%
3.0%
Ecuador
$2.4
$60.7
2441.4%
0.4%
2.2%
United
$6.6
$52.0
688.3%
1.2%
1.9%
Kingdom
Peru
$2.6
$49.3
1819.4%
0.5%
1.8%
All Others
$199.0
$782.6
293.3%
35.0%
28.6%
World
$568.9
$2,737.4
381.2%
100.0%
100.0%
Source: China Customs (downloaded via Trade Data Monitor). Categories based on the 6-digit product codes outlined by the World Customs Organization in “HS Classification Reference for Covid-19 Medical Supplies,” ed. 2, as updated April 30, 2020. Notes: Changes in the value of trade are determined by both the quantity of trade and the price of the good. Many of these products experienced an increase in both price and quantity, during the March-July 2020 period.
Congressional Research Service
23
COVID-19: China Medical Supply Chains and Broader Trade Issues
In defining these product groups, CRS has attempted to capture the most common HS categorizations for these items, however some trade of these items may have been exported under additional HS codes not reflected here, and, conversely, the product groups are not exclusive to Covid-19-related gear and may include other similar trade. See footnotes for specific product classifications. EU-27 includes the 27 member states of the European Union, excluding the United Kingdom. U.A.E. is the United Arab Emirates. a. HS 630790 is a higher-level classification that includes N95 masks among other types of “Made-Up Textile
Articles [not classified elsewhere].”
b. Other Face and Eye PPE is defined as HS 392690, 481890, 900490, and 902000. c. Gowns and Other PPE are defined as HS 392620, 401590, 481850, 621010, 621040, 621050, and 650500. d. PPE Gloves are defined as HS 392620, 401511, 401519, 611610, and 621600. e. Covid-19 Test Kits and Instructions are defined as HS 300215, 382100, 382200, and 902780.
U.S. Shortages
U.S. national and state-level health authorities began reporting shortages of medical supplies—including PPE such as gowns and face masks—sincein February. On March 18, President Trump issued Executive Order 13909, Prioritizing and Allocating Health and Medical Resources to Respond to the Spread of COVID–19, which announced the President's invocation of the Defense Production Act of 1950 (DPA) in response to the COVID-19 pandemic.4156 The DPA confers broad presidential authorities to mobilize domestic industry in service of the national defense, defined in statute as various military activities and "homeland security, stockpiling, space, and any directly related activity" (50 U.S.C. §4552), including emergency preparedness activities under the Stafford Act, which has been used for public health emergencies.4257 Among other authorities, Title I of the DPA allows the President to require persons (including businesses and corporations) to (1) prioritize and accept government contracts for materials and services, and (2) allocate or control the general distribution of materials, services, and facilities as necessary to promote the national defense. The Administration, however, is only publicly providing limited direction to the private sector under this authority.43
58
Any potential use of the DPA to respond to the COVID-19 pandemic may require some amount of time to produce adequate supplies, considering the large volumes of products, particularly PPE and ventilators, which are currentlywere in urgent demand. Many U.S. firms are hesitant to invest in substantial increases in production, including obtaining the capital equipment and other inputs required, until they have a guaranteed buyer and price.4459 Manufacturing firms, such as General Motors, Ford Motor Company, and Tesla are repurposingrepurposed factory production for ventilators, but
56 Executive Order 13909 “Prioritizing and Allocating Health and Medical Resources to Respond to the Spread of COVID-19,” 85 Federal Register 56, March 23, 2020, https://www.govinfo.gov/content/pkg/FR-2020-03-23/pdf/2020-06161.pdf; CRS Report R43767, The Defense Production Act of 1950: History, Authorities, and Considerations for Congress, by Michael H. Cecire and Heidi M. Peters; CRS Insight IN11231, The Defense Production Act (DPA) and COVID-19: Key Authorities and Policy Considerations, by Michael H. Cecire and Heidi M. Peters; CRS Insight IN11280, COVID-19: Industrial Mobilization and Defense Production Act (DPA) Implementation, by Michael H. Cecire and Heidi M. Peters.
57 CRS Insight IN11231, The Defense Production Act (DPA) and COVID-19: Key Authorities and Policy Considerations, by Michael H. Cecire and Heidi M. Peters
58 The White House, “Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing,” March 22, 2020, https://www.whitehouse.gov/briefings-statements/remarks-president-trump-vice-president-pence-members-coronavirus-task-force-press-briefing-8/.
59 Lena H. Sun and Rachel Siegel, “As Demand Spikes for Medical Equipment, This Texas Manufacturer is Caught in the Coronavirus Supply Chain Panic, The Washington Post, February 15, 2020, https://www.washingtonpost.com/business/2020/02/15/coronavirus-mask-shortage-texas-manufacturing/.
Congressional Research Service
24
link to page 30 COVID-19: China Medical Supply Chains and Broader Trade Issues
factory production for ventilators, but defense logistics experts expectexpected this effort to take months.4560 Additionally, in the United States, PPE and ventilators for use in the health care setting are considered medical devices and require marketing permission from the U.S. Food and Drug Administration (FDA).
The Trump Administration'’s relatively late formal invocation and activation of the DPA, which could effectively serve as an export constraint on U.S.-produced medical supplies, arguably left discretion to U.S. companies to decide whether to fill export or domestic orders first. By contrast, governments in Taiwan, Thailand, France, and Germany boosted production but restricted exports, further curtailing U.S. supply options. In January and February 2020, organizers of U.S. private sector relief efforts reportedly purchased large amounts of U.S. PPE products for airlift to China, further depleting U.S. supplies.46
61
Some Members of Congress have called for broader tariff relief or at least new exclusions for existing tariffs and a moratorium on any new tariffs. Other Members and U.S. domestic producers argue that such liberalization could open the U.S. market to a flood of imports during an economic downturn.4762 The Office of the United States Trade Representative (USTR) announced on March 6, 2020, that it would lift tariffs imposed under Section 301 authorities on 19 specific products and 8 10-digit subheadings of medical supply and equipment items from China (See Table 5).63
The USTR announced on March 20, 2020, that, prior to the COVID-19 outbreak, the agency had been working with the U.S. Department of Health and Human Services “to ensure that critical medicines and other essential medical products were not subject to additional Section 301 tariffs.” Consequently, the United States had not imposed tariffs on certain critical products, such as ventilators, oxygen masks, and nebulizers. The USTR indicated that, in recent months, it has prioritized the review of requests for exclusions on medical care products, resulting in exclusions granted on basic medical supplies, including gloves, soaps, facemasks, surgical drapes, and hospital gowns.
Since March 2020, the USTR has exempted certain medical products from Section 301 tariffs in several rounds of exclusions. CRS could not determine exactly how many of them have been exempted on the basis of COVID-19 concerns, as the USTR does not specify the rationale for
60 Aaron Gregg, Dan Lamothe, and Christian Davenport, “Having Automakers Churn Out Ventilators Won’t be Quick or Easy, Experts Say,” The Washington Post, March 21, 2020. 61 U.S. Embassy & Consulates in China, “The United States Announces Assistance to the COVID-19,” U.S. Department of State, press release, February 7, 2020, https://china.usembassy-china.org.cn/the-united-states-announces-assistance-to-the-novel-coronavirus/; UPS Foundation, “UPS to Airlift More Than 2 Million Masks and
Protective Gear To China,” UPS Pressroom, press release, January 31, 2020, https://pressroom.ups.com/pressroom/ContentDetailsViewer.page?ConceptType=PressReleases&id=1580479415168-269&WT.mc_id=UPSCOM_NEWSANDINFO_CORONA_PRESSRELEASE_013120; Direct Relief, “Direct Relief Rushes Facial Masks to China to Fight Coronavirus Spread,” press release, January 28, 2020, https://www.directrelief.org/2020/01/direct-relief-rushes-facial-masks-to-china-to-fight-coronavirus-spread/; Tad Walch, “Inside the church’s donation of masks, coveralls and goggles to China over the coronavirus outbreak,” Deseret News, January 29, 2020, https://www.deseret.com/faith/2020/1/29/21113386/coronavirus-china-outbreak-lds-church-mormon-russell-nelson-donation-chinese-health-wuhan. 62 William Mauldin, “U.S. to Allow Some Delays in Tariff Payments,” The Washington Post, March 26, 2020. 63 “Notice of Product Exclusions: China’s Acts, Policies and Practices Related to Technology Transfer, Intellectual Property, and Innovation,” USTR Notice, U.S. Federal Register, March 10, 2020, https://www.federalregister.gov/documents/2020/03/10/2020-05000/notice-of-product-exclusions-chinas-acts-policies-and-practices-related-to-technology-transfer. Also see https://www.govinfo.gov/content/pkg/FR-2020-03-10/pdf/2020-05000.pdf and https://ustr.gov/sites/default/files/enforcement/301Investigations/%24300_Billion_Exclusions_Granted_March_2020.pdf.
Congressional Research Service
25
COVID-19: China Medical Supply Chains and Broader Trade Issues
granting exclusions in its announcements. While CRS can identify some products, there are others with known or potential medical uses—or inputs for the manufacture thereof—that have received exclusions but whose ultimate purpose cannot always be ascertained from HTSUS subheadings or the provided product descriptions (e.g., organic chemicals or textiles for the manufacture of pharmaceuticals or PPE).
In addition, at the end of March 2020, the USTR published a Federal Register notice seeking comments to determine if further modifications to the Section 301 tariffs on U.S. imports from China are necessary to respond to the COVID-19 pandemic in the United States. The notice provided no further guidance on the types of products that the USTR considers to be “medical-care products.” The review of comments is to run parallel to, and is not to affect, any ongoing product exclusion requests still under review. The USTR has not indicated what form the response would take or when it will respond to comments—only that it will review them on a rolling basis. These comments may already be informing product exclusion decisions, or may lead to the establishment of a new formal exclusion process, akin to that used for Lists 3 and 4, but strictly for medical products.
Table 4).48
The Administration appears reluctant to liberalize non-health related tariffs, preferring to delay tariff payments instead.4964 In late March 2020, the U.S. Customs and Border Protection sent notices to companies saying that officials will approve some delays in tariff payments to offer economic relief due to the severity of COVID-19; they may also be weighing a broader suspension of collecting duties.5065 Separate from COVID-19, with regard to existing tariff exemptions, on March 20, USTR invited industry to submit public comments beginning on April 20, regarding whether USTR should extend certain tariff exclusions on other products already granted in June 2019 that expire in June 2020.5166 A broader liberalization of U.S. tariffs on Chinese goods during the COVID-19 outbreak, could further expose the U.S. economy to Chinese excess industrial capacity at a point of economic downturn in the United States. Chinese firms also could capture market share and gain a unique foothold in the U.S. market through market softening and if the United States were to relax FDA and other product certifications.
Table 45. Recent Section 301 Tariff Exclusions on Select U.S. Imports from China
Value (U.S. Dollars) and Share of U.S. Imports (%) in 2019
China’s
Value of
Value of U.S.
Share of
Total U.S.
Imports
Total U.S.
HTS
Imports
from China
Imports
Number
Description
(US$)
(US$)
(%)
3401.19.0000
Soap and Organic Surface-Active Products
164,333,744
106,484,402
64.8
3926.90.9910*
Laboratory Ware
485,245,133
86,823,074
17.9
4015.19.0510
Nitrile and Sterile Gloves
147,518,639
12,889,989
8.7
64 Robert E. Lighthizer, “Lighthizer Responds: Medical Trade Tariffs,” Letter to the Editor, The Wall Street Journal, March 21-22, 2020.
65 Alex Leary and William Mauldin, “Import Tariffs Will be Halted, Officials Say,” The Wall Street Journal, March 28-29, 2020.
66 “Request for Comments Concerning the Extension of Particular Extensions Granted Under the June 2019 Product Exclusion Notice From the $34 Billion Action Pursuant to Section 301: China’s Acts, Policies and Practices Related to Technology Transfer, Intellectual Property and Innovation,” USTR Notice, U.S. Federal Register, March 20, 2020, https://www.federalregister.gov/documents/2020/03/20/2020-05890/request-for-comments-concerning-the-extension-of-particular-exclusions-granted-under-the-june-2019.
Congressional Research Service
26
COVID-19: China Medical Supply Chains and Broader Trade Issues
China’s
Value of
Value of U.S.
Share of
Total U.S.
Imports
Total U.S.
HTS
Imports
from China
Imports
Number
Description
(US$)
(US$)
(%)
4015.19.0550
Nitrile and Sterile Gloves
1,363,144,838
200,159,326
14.7
4818.90.0000
Disposable (Paper) Household, Sanitary, or
476,012,068
356,642,980
74.9
Hospital Bed Articles
6210.10.5000
Personal Protective Equipment
795,151,929
426,658,153
53.7
6307.90.6090
Medical Protective Clothing
11,786,110
5,502,995
46.7
6307.90.6800
Medical Protective Clothing
445,250,763
159,699,946
35.9
6307.90.9889*
Personal Protective Equipment
3,209,428,978
2,307,838,576
71.9
9004.90.0000*
Protective Goggles
919,198,348
503,787,243
54.8
Source:Value (U.S. Dollars) and Share of U.S. Imports (%) in 2019
|
HTS Number |
Description |
Value of Total U.S. Imports (US$) |
Value of U.S. Imports from China (US$) |
China's Share of Total U.S. Imports (%) |
|
3401.19.0000 |
Soap and Organic Surface-Active Products |
164,333,744 |
106,484,402 |
64.8 |
|
3926.90.9910* |
Laboratory Ware |
485,245,133 |
86,823,074 |
17.9 |
|
4015.19.0510 |
Nitrile and Sterile Gloves |
147,518,639 |
12,889,989 |
8.7 |
|
4015.19.0550 |
Nitrile and Sterile Gloves |
1,363,144,838 |
200,159,326 |
14.7 |
|
4818.90.0000 |
Disposable (Paper) Household, Sanitary, or Hospital Bed Articles |
476,012,068 |
356,642,980 |
74.9 |
|
6210.10.5000 |
Personal Protective Equipment |
795,151,929 |
426,658,153 |
53.7 |
|
6307.90.6090 |
Medical Protective Clothing |
11,786,110 |
5,502,995 |
46.7 |
|
6307.90.6800 |
Medical Protective Clothing |
445,250,763 |
159,699,946 |
35.9 |
|
6307.90.9889* |
Personal Protective Equipment |
3,209,428,978 |
2,307,838,576 |
71.9 |
|
9004.90.0000* |
Protective Goggles |
919,198,348 |
503,787,243 |
54.8 |
Source: CRS using information from the Office of the U.S. Trade Representative (85 FR 13970 and 85 FR 15244) and data from the U.S. International Trade Commission'’s DataWeb.
Notes: *Only a portion of the HTS ten-digit subheading is covered by the tariff exclusion. For more detail, see 85 FR 15244. N95 and other protective masks have historically been classified under tariff subheading 6307.99.9889, which includes other miscellaneous textile article made from similar materials.
In an effort to quickly bring overseas medical supplies into the United States, the Federal Emergency Management Agency (FEMA), announced on March 29, 2020 that it was arranging airlift for 22 flights, most from Asia, over the subsequent two weeks. The airlift iswas for medical supplies that medical distributors already planned to import into the United States, but it acceleratesaccelerated their delivery arrival time by shipping by air instead of ocean freight.52
67 As of May 2020, FEMA said it had coordinated the delivery of more than 97 million respirators, 133.7 million surgical masks, and 10,600 ventilators, among other critical medical supplies.68
Separate from medical supplies specific to COVID-19, a longer-term disruption of China's ’s pharmaceutical and medical exports could increase the cost of everyday drugs and routine medical procedures in the United States. This could happen as it becomes harder to import APIs for common drugs and components for medical devices. According to FDA officials, in 2018, China ranked second among countries that export drugs and biologics to the United States by import line (accounting for 13.4% of U.S. imports of those products).5369 However, FDA states it is not able to determine the volume of APIs that China is manufacturing, given the complexity of the supply chain and gaps in what pharmaceutical companies are required to disclose about their inputs.5470 China is also a leading supplier of APIs in global supply chains for painkillers, diabetes
67 Jonathan Swan and Joann Muller, “Inside the Start of the Great Virus Airlift,” Axios, March 29, 2020, https://www.axios.com/coronavirus-airlift-masks-medical-supplies-1d1913bf-744e-41cf-895c-d8934afa2c36.html?utm_source=newsletter&utm_medium=email&utm_campaign=newsletter_axiossneakpeek&stream=top.
68 Federal Emergency Management Agency, “FEMA Releases State-by-State PPE Data,” press release, May 14, 2020. 69 Testimony of FDA Associate Commissioner for Global Policy and Strategy Mark Abdoo, in U.S. China Security and Economic Review Commission, Exploring the Growing Reliance on China’s Biotech and Pharmaceutical Products, July 31, 2019, https://www.fda.gov/news-events/congressional-testimony/exploring-growing-us-reliance-chinas-biotech-and-pharmaceutical-products-07312019. Information provided by FDA’s Office of Legislation through personal communication with CRS. FDA’s usage of the term “import line” refers to a distinct regulated product within a shipment through customs.
70 FDA, Testimony of Dr. Janet Woodcock, Director the Center for Drug Evaluation and Research, “Securing the U.S.
Congressional Research Service
27
COVID-19: China Medical Supply Chains and Broader Trade Issues
China is also a leading supplier of APIs in global supply chains for painkillers, diabetes medicines, and antibiotics, meaning a slowdown in API exports from China could increase cost pressures faced by U.S. drug manufacturers.5571 For example, China accounts for 52% of U.S. imports of penicillin, 90% of tetracycline, and 93% of chloramphenicol.5672 On February 27, FDA Commissioner Stephen Hahn announced that a manufacturer of an unspecified human drug informed FDA of a shortage the drug'’s supply related to a Chinese API manufacturer affected by COVID-19.5773 Because information disclosed to FDA regarding drug shortages is considered proprietary, FDA did not disclose the name of the drug in question, but did note that alternatives exist for patient use.58
China'74 According to congressional testimony delivered in October 2019 by Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, the FDA is unable to estimate the total volume of all API produced in China. Woodcock also stated in her testimony that the FDA was able to identify three drugs on the WHO’s Essential Medicines list whose API manufacturers are based only in China: capreomycin and streptomycin, commonly used to treat tuberculosis, and sulfadiazine, used to treat chancroid and trachoma.75
China’s role as the primary supplier of APIs to global manufacturers of generic pharmaceuticals, particularly in India, is likely to increase overall costs of generic pharmaceuticals for consumers in the United States in the short-to-medium term. The outbreak of COVID-19 in India could also affect the availability of generic pharmaceuticals in the United States. India, which supplies approximately 40% of generic pharmaceuticals used in the United States, imports nearly 70% of its APIs from China.5976 In March 2020, India imposed export restrictions on several drugs whose supply chains rely on China, leading to fears of potential global shortages of generic drugs that have since escalated after India announced a nationwide 21-day lockdown.60
77 Additionally, further tensions in China-India bilateral trade resulting from the ongoing border conflict between the two countries could pose further risk to generic pharmaceutical supply chains.
Global Trade Restrictions Global Trade Restrictions
Amid concerns about the availability of personal protective equipment (PPE), medical supplies, and pharmaceuticals, a growing number of nations have appliedapplied temporary export controls and other restrictions on the overseas sales of these products. While export controls do not necessarily
Drug Supply Chain: Oversight of FDA’s Foreign Inspection Program,” December 10, 2019. 71 Stephanie Findlay, Hannah Kuchler, and Sarah Neville, “Drugmakers braced for coronavirus disruption to China supplies,” Financial Times, February 12, 2020, https://www.ft.com/content/8630c51c-4cc0-11ea-95a0-43d18ec715f5; Reuters, “China Virus Outbreak Threatens Global Drug Supplies: European business group,” February 17, 2020, https://www.reuters.com/article/us-china-health-pharma-antibiotics/china-virus-threatens-global-antibiotics-supply-european-business-group-idUSKBN20C08S.
72 CRS calculations using data from the U.S. International Trade Commission’s DataWeb (HTSUS 2941.30 and 2941.10).
73 U.S. Food and Drug Administration, “Coronavirus (COVID-19) Supply Chain Update,” Press release, February 27, 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update.
74 Food and Drug Administration, “Frequently Asked Questions about Drug Shortages,” last updated July 05, 2018, https://www.fda.gov/drugs/drug-shortages/frequently-asked-questions-about-drug-shortages#q7; and CDER Office of Compliance Office of Drug Security, Integrity & Recalls, “FDA Compliance Focal Point for Imports & Exports of CDER Regulated Drugs, https://www.fda.gov/media/91681/download. 75 FDA, Testimony of Dr. Janet Woodcock, Director the Center for Drug Evaluation and Research, “Securing the U.S. Drug Supply Chain: Oversight of FDA’s Foreign Inspection Program,” December 10, 2019. 76 Yanzhong Huang, “The Coronavirus Outbreak Could Disrupt the U.S. Drug Supply,” Council on Foreign Relations, March 5, 2020, https://www.cfr.org/in-brief/coronavirus-disrupt-us-drug-supply-shortages-fda; Julianna Tatelbaum, “Fears of US drug shortages grow as India locks down to curb the coronavirus,” CNBC, March 24, 2020. 77 Reuters, “Global supplier India curbs drug exports as coronavirus fears grow,” March 3, 2020.
Congressional Research Service
28
COVID-19: China Medical Supply Chains and Broader Trade Issues
restrictions on the overseas sales of these products. While export controls do not necessarily prohibit export activity, they make export licenses a requirement, which could lead to transactions being delayed and potentially denied or cancelled. As medical professionals around the world scramblescrambled to find gloves, face shields, protective garments, disinfectants, ventilators, and other equipment needed to fight COVID-19, these measures are highlighting the risks—and exacerbating the challenges—of relying on complex global supply chains and distribution channels.61channels that are not sufficiently diversified.78 World Trade Organization (WTO) rules prohibit export bans except for rare instances in which a member invokes a measure citing national security concerns. In an effort to promote transparency, the WTO is publishing a list of temporary export bans that countries are enacting during COVID-19 and notifying to the WTO.6279 On March 30, 2020, the G-20 issued a joint statement that emphasized the importance of keeping markets open and ensuring the adequate production and fair and equitable distribution of medical products to where they are most needed. The statement emphasized that any measures a country might adopt to protect health should be targeted, proportionate, transparent, and temporary.63
80
So far this year, China and more than 24 other economies, including India and, more recently, the European Union,6481 have imposed either limits or formal or de facto bans on certain exports.65 82 Many of the existing and proposed measures could restrict access to markets on which the United States depends for certain imports. These include medical ventilators (for which Singapore and China accounted for 35% and 17%, respectively, of U.S. imports in 2019), breathing and gas masks (France, the United Kingdom, and Italy, 47% combined), CT scanners (Germany, 50%), medical protective equipment of textile materials (China, 72%), digital and infrared thermometers (China, 36%), pharmaceuticals (Ireland, Germany, Switzerland, and Italy, 53% combined), and tetracycline and penicillin (China, 90% and 52%, respectively).66
83 Many governments rescinded these temporary measures after the height of the COVID19 outbreak in their countries. China did not provide notice of its de facto export constraints that redirected supply for domestic use to the World Trade Organization (WTO), as other countries did.84 China Customs Delays Release of January-February 2020 Trade Data
The Chinese government released top-level import and export figures to the media in early March 2020, but China Customs did not release its detailed January-February 2020 monthly trade data, as scheduled on March 19, 2020 until 11 days later on March 30, 2020. This data is important in providing additional details on trade patterns
78 Bryce Baschuk, “Export Bans on Medical Supplies to Hamper Global Virus Response,” Bloomberg, March 18, 2020. Some public health officials and trade experts have also expressed concern that export controls and other restrictions could reduce incentives for companies to ramp up production. See, for example, James Politi, Aime Williams, and Clive Cookson, “US official hits out at hoarding of coronavirus medical supplies,” Financial Times,” March 5, 2020. 79 World Trade Organization, “COVID-19 and World Trade,” last updated March 31, 2020, https://www.wto.org/english/tratop_e/covid19_e/covid19_e.htm.
80 G20 Leaders’ Statement, “Extraordinary G20 Leaders Summit Statement on COVID-19,” March 30, 2020, https://g20.org/en/media/Documents/G20_Extraordinary%20G20%20Leaders%E2%80%99%20Summit_Statement_EN%20(3).pdf.
81 Lili Bayer, Jillian Deutsch, Jakob Hanke Vale, and Paola Tamma, “EU Moves To Limit Exports of Medical Equipment Outside the Bloc,” Politico, March 15, 2020. 82 Simon J. Evenett, Tackling Coronavirus: The Trade Policy Dimension, Swiss Institute of International Economics and Department of Economics, University of St. Gallen, Switzerland, March 10, 2020. Some countries, including Australia, Brunei, Canada, Chile, Myanmar, New Zealand and Singapore, have pledged “to keep trade lines open.” Andrea Shalal, “U.S. Should Refrain from Export Controls in Pandemic Response: Chamber of Commerce,” Reuters, March 25, 2020.
83 CRS calculations using data from the U.S. International Trade Commission’s DataWeb and Global Trade Atlas. 84 World Trade Organization, “COVID-19: Trade and trade-related measures,” last updated July 31, 2020, https://www.wto.org/english/tratop_e/covid19_e/trade_related_goods_measure_e.htm.
Congressional Research Service
29
COVID-19: China Medical Supply Chains and Broader Trade Issues
|
China's Economic Recovery: Prospects and Implications
China'
Domestic Supply: U.S. vs Foreign Made Products
The COVID-19 pandemic exposed the gaps in U.S. domestic industry and trade data, complicating the ability to assess U.S. domestic capabilities in relation to global supply and trade dependencies for critical products in a time of crisis.86 In general, the U.S. government does not keep records of the domestic production of specific, individual items (e.g., surgical masks and gloves) by quantity or value—and the U.S. government also does not track how much of this production is ultimately destined for the U.S. market.87 Of what the United States produces domestically, the U.S. government tracks categories of products that are exported to foreign markets. It also collects statistics for broad industry sectors, such as gross output, value added—also known as gross domestic product (GDP) by industry—and intermediate inputs.88 Another complicating factor in the analysis of U.S. production—and U.S. reliance on imports—of PPE and medical products is that there are no domestic or internationally agreed guidelines, standards, or definitions of what specific products make up these categories. Therefore, questions such as “how much PPE does the United States currently produce relative to what it imports?” or “by how much has domestic production of pharmaceuticals increased since the COVID-19 outbreak?” are difficult to answer. 89
However, data from an annual government survey of U.S. manufacturers, analyzed in conjunction with official U.S. trade statistics, may give a partial insight into some domestic production activities and provide a rough estimate of the share that imported PPE and medical products account for of total U.S. supply.
Annual Survey of Manufactures and Trade Statistics The U.S. Census Bureau’s Annual Survey of Manufactures (ASM) measures current U.S. manufacturing activity such as industry outputs, inputs, and operating status.90 It provides sample
85 Chen Aizhu and Tom Daly, “Where’s the Data? Angst for Commods Traders as China Trade Figures Held in Limbo,” Reuters, June 1, 2018, https://www.reuters.com/article/us-usa-trade-china-data/wheres-the-data-angst-for-commods-traders-as-china-trade-figures-held-in-limbo-idUSKCN1IX4LQ.
86 For more detail, see CRS In Focus IF11648, Medical Supply Chains and Policy Options: The Data Challenge, by Andres B. Schwarzenberg and Karen M. Sutter.
87 The U.S. Department of Commerce, for example, collects more information than it makes publicly available due to confidentiality requirements (e.g., 13 U.S.C § 9 and 15 C.F.R. § 801.5). However, that information does not include details on specific items produced by manufacturing establishments.
88 For more detail, see U.S. Bureau of Economic Analysis, “Industry Economic Accounts,” at https://www.bea.gov/data/economic-accounts/industry. However, quantity and value information of total U.S. production is not available at the item level.
89 See CRS In Focus IF11648, Medical Supply Chains and Policy Options: The Data Challenge, by Andres B. Schwarzenberg and Karen M. Sutter
90 U.S. Census Bureau, “Annual Survey of Manufactures (ASM),” at https://www.census.gov/econ/overview/ma0300.html.
Congressional Research Service
30
link to page 35 COVID-19: China Medical Supply Chains and Broader Trade Issues
estimates of statistics for manufacturing establishments in the United States based on the North American Industry Classification System (NAICS).91
The ASM statistics include the value added by manufacturing, total value of shipments for close to 1,400 classes of manufactured products, costs of materials, and inventories. NAICS categories capture most, but not necessarily all, establishments producing PPE and medical products. However, there is a time lag with the data, which presents challenges in developing a real time picture of the industry, as 2018 is the most recent year for which data are available.92
In addition, the U.S. Bureau of Economic Analysis (BEA) and the Census Bureau collect data on U.S. imports on a monthly, quarterly, and yearly basis.93 Using these data, and matching them with the ASM, CRS was able to obtain a rough estimate of the imported—and thereby infer the domestic—share of U.S. supply for categories considered to include PPE, pharmaceuticals, and other medical-related products in 2018 (Table 6). The figures were calculated at the NAICS 6-digit subheading level—the most disaggregated level for which NAICS data are available. However, because these are broad categories, the data may underestimate or overestimate actual domestic production and imports.94
Estimates suggest that the United States seems to be heavily dependent on certain imports (for more than 90% of domestic supply in some cases). However, foreign source dependence varies by product category. In 2018, the United States imported many low-end and labor-intensive manufactured products primarily from China (e.g., apparel from fabric such as hospital gowns). Many of the higher value added and skill-intensive imported products, on the other hand, came mainly from Europe (e.g., irradiation machines and biological products such as vaccines).
Table 6. Estimate of the Imported Share of U.S. Domestic Supply: Select Medical-
Related Manufactured Good Categories in 2018
Share of Domestic Supply (%)
Total
U.S. Imports
U.S.
Supply
from
Imports
NAICS
Import
European
from
Code
Description
s
Union
China
315220 Men's and Boys' Cut and Sew Apparel
98
3
20
[apparel from fabric, including hospital/medical/laboratory service apparel]
315240 Women's, Girls', and Infants' Cut and Sew Apparel
96
3
36
[apparel from fabric, including hospital/medical/laboratory service apparel]
333314 Optical Instruments and Lenses
94
14
23
[microscopes, telescopes, prisms, and lenses; coating or polishing lenses; and mounting lenses]
91 For more detail on NAICS, see U.S. Census Bureau, “North American Industry Classification System: Introduction to NAICS,” at https://www.census.gov/eos/www/naics/.
92 U.S. Census Bureau, “Annual Survey of Manufactures: Summary Statistics for Industry Groups and Industries in the U.S.: 2018.” 93 U.S. Department of Commerce, U.S. Bureau of Economic Analysis (“International Transactions” and “International Trade in Goods and Services”) and U.S. Census Bureau (“Foreign Trade”). 94 U.S. import statistics include imports of goods from U.S.-owned affiliates abroad.
Congressional Research Service
31
COVID-19: China Medical Supply Chains and Broader Trade Issues
Total
U.S. Imports
U.S.
Supply
from
Imports
NAICS
Import
European
from
Code
Description
s
Union
China
325414 Biological Products (Except Diagnostic)
79
59
*
[vaccines, toxoids, blood fractions, and culture media of plant or animal origin, except diagnostic]
339115 Ophthalmic Goods
60
22
20
[prescription eyeglasses, contact lenses, sunglasses, eyeglass frames, reading glasses made to standard powers, and protective eyewear]
313210 Broadwoven Fabrics
55
10
17
[fabrics and felts, including surgical gauzes]
325411 Medicinal and Botanical Drugs and Vitamins
48
34
8
[uncompounded medicinal chemicals and their derivatives and botanicals]
325413 In-Vitro Diagnostic Substances
48
27
3
[chemical, biological, or radioactive diagnostic substances]
325199 All Other Basic Organic Chemicals
42
14
9
[isopropyl alcohol and glycerin]
334517 Irradiation Apparatus
41
25
4
[beta-rays, gamma-rays, X-rays, or other ionizing radiation apparatus]
339113 Surgical Appliances and Supplies
39
15
6
[orthopedic devices, prosthetic appliances, surgical dressings, crutches, surgical sutures, personal industrial safety devices]
325412 Pharmaceutical Preparations
39
23
*
[in-vivo diagnostic substances and pharmaceutical preparations]
339112 Surgical and Medical Instruments
36
10
2
[syringes, needles, anesthesia apparatus, blood transfusion equipment, catheters, surgical clamps, and medical thermometers]
Source: CRS analysis with data from the U.S. Census Bureau and the U.S. International Trade Commission. Notes: (1) Shares are rough estimates calculated at the NAICS 6-digit subheading level, which may cover products that are not for medical use; (2) 2018 is the most recent year for which annual data from the ASM are available; (3) * = Share of domestic supply is less than 0.05%; and (4) descriptions in brackets are only select examples of products or items covered by the NAICS subheading.
This picture, however, may have changed between 2018 and 2020 as China capitalizes on the investments that the government has made in recent years to push ahead on ambitious state-led programs such as Made in China 2025 (MIC2025).95 One of the goals of MIC2025 is to modernize the Chinese economy and turn China into a global leader in the manufacturing of
95 For more detail on MIC2025, see CRS In Focus IF10964, “Made in China 2025” Industrial Policies: Issues for Congress, by Karen M. Sutter
Congressional Research Service
32
COVID-19: China Medical Supply Chains and Broader Trade Issues
biopharmaceuticals and high-performance medical devices. Lack of data and other constraints limit the U.S. ability to assess in real time the progress of these efforts and their impact on the U.S. economy and industrial base.
Other Sources of Data and Information Sizing up the U.S. government’s reliance on foreign goods faces similar challenges in data limitations.96 The U.S. General Services Administration (GSA) maintains a database, the Federal Procurement Data System-Next Generation (FPDS-NG, or FPDS), where federal agencies are required to report procurement contracts whose estimated value is $10,000 or more.97 The procurement data in FPDS-NG are not fully reliable. There are documented quality issues documented relating to accuracy, completeness, and timeliness of its data.98 These limitations have prompted many analysts to rely on FPDS-NG data primarily to identify broad trends and produce rough estimates, or to gather information about specific contracts. With these limitations in mind, FPDS-NG data may provide general information regarding the value, quantity, and types of domestic and foreign-made goods hat U.S. government agencies procure.
Other information on domestic capacity, as well as changes resulting from increased production in the aftermath of the COVID-19 outbreak, generally comes from private research firms, news outlets, and trade associations. Many of the figures cited are often based on surveys, firms’ press releases, or firms/industries’ forecasts, which may differ significantly from actual production.
China’s Economic Recovery: Prospects and Implications China’s leaders are focusing on resuming manufacturing production to jumpstart economic growth. 68 99 At an executive session of China'’s cabinet, the State Council, on March 17, Chinese officials emphasized the importance of stabilizing employment and announced that the government would streamline business approvals and fast-track approvals for large infrastructure projects. They also offered government support to alleviate shortages of labor, raw materials, funds, and protective gear.69100 To facilitate economic activity, the Chinese government also appears to be liberalizing company health requirements and lifting intra-provincial and intra-city travel and transportation restrictions. NDRC spokesperson Meng Wei said on March 17, 2020 that transportation was operating normally. Zhejiang, Jiangsu, and Shanghai were operating at close to 100% of normal capacity; and over 90% of large-scale industrial companies outside of Hubei had resumed production.70
96 For more detail on the role of international trade in U.S. government procurement, see CRS In Focus IF11580, U.S. Government Procurement and International Trade, by Andres B. Schwarzenberg.
97 Although it is not material for the immediate purposes of this report, the primary federal procurement reporting tool is scheduled to move from FPDS to the System for Award Management (SAM) in October 2020.
98 For more information on FPDS-NG data quality issues, see “Appendix A. FPDS Background, Accuracy Issues, and Future Plans” in CRS Report R44010, Defense Acquisitions: How and Where DOD Spends Its Contracting Dollars, by John F. Sargent Jr. and Christopher T. Mann.
99 Ryan Woo, Se Young Lee, David Stanway, and Andrew Galbraith, “Goldman Sees China’s Economy Shrinking 9 Percent in First Quarter Amid Coronavirus Outbreak,” Reuters, March 16, 2020, https://www.reuters.com/article/us-health-coronavirus-china-toll/goldman-sees-chinas-economy-shrinking-9-in-first-quarter-amid-coronavirus-outbreak-idUSKBN21340T.
100 Hua Xia, “China Advances Streamlining Approval Procedures, Fostering New Growth Drivers to Keep Employment Stable,” Xinhua, March 17, 2020, http://www.xinhuanet.com/english/2020-03/17/c_138888715.htm.
Congressional Research Service
33
COVID-19: China Medical Supply Chains and Broader Trade Issues
resumed production.101 Company reports of opening and resumption of operations may not mean that these facilities are fully online or operating at pre-crisis levels, however. Several economic analysts and news outlets, including the Financial Times, have published alternative measures of business resumption rates using proxies for economic activity—such as data on traffic congestion, air pollution levels, and container freight movement. Overall, many of these measures suggest that businesses across China are not returning to full capacity at the rates being reported by local and provincial governments.71102 In Wuhan, the center of the original outbreak, the Hubei provincial government issued a notice in March—that applies to Wuhan as Hubei'’s capital—allowing certain companies to resume work ahead of other production. This included companies in the medical and health industry, as well as companies producing protective gear, disinfectant, daily necessities, agriculture, and products critical to national and global supply chains.72
103 China Positioning to Export
China'’s economy depends on exports and the foreign exchange it earns through exports, as well as on the large productive role that foreign firms play in the domestic market and as exporters. Seeking to stabilize drops in foreign investment and trade, on March 12, Commerce Vice Minister Wang Shouwen held a call with 400 members of the American Chamber of Commerce in China, and on March 13, he held a similar webinar with the European Chamber of Commerce in China's ’s Advisory Council. Vice Minister Wang pressed companies to reopen operations and increase investments in China. Other Chinese agencies represented included NDRC, MIIT, the National Health Commission, the General Administration of Drug Supervision, the State Administration for Market Regulation, the General Administration of Customers, the Civil Aviation Administration of China, the Ministry of Transportation, and the State Taxation Administration.73
104
During past crises, such as the global financial crisis of 2008-09, China has pressed firms to idle facilities and keep them production-ready (instead of shuttering them) and retain workers (instead of laying them off) to maintain social stability and facilitate efforts to quickly ramp up production and exports later. 74 105 These stimulus efforts are sometimes less visible than fiscal policies in other countries. Several market watchers have noted that, while a 17% drop in Chinese exports in January-February 2020 is significant, it is not as dramatic when considering China's economy ’s economy
101 Ryan Woo, Se Young Lee, David Stanway, and Andrew Galbraith, “Goldman Sees China’s Economy Shrinking 9 Percent in First Quarter Amid Coronavirus Outbreak,” Reuters, March 16, 2020, https://www.reuters.com/article/us-health-coronavirus-china-toll/goldman-sees-chinas-economy-shrinking-9-in-first-quarter-amid-coronavirus-outbreak-idUSKBN21340T.
102 John Burn-Murdoch, Cale Tilford, Steven Bernard, Keith Fray, and Alan Smith, “Coronavirus Economic Tracker: Latest Global Fallout,” Financial Times, accessed March 26, 2020; and “Coronavirus: Getting China Back to Work,” Trivium China, last updated April 1, 2020, https://triviumchina.com/2020/03/07/coronavirus-getting-china-back-to-work/.
103 Li Yan, “Wuhan Companies Begin to Resume Production,” China Daily, March 17, 2020, http://www.ecns.cn/m/business/2020-03-17/detail-ifzunmih1236408.shtml.
104 “MOFCOM VM Wang Shouwen Holds Back to Work Call,” American Chamber of Commerce in China, March 19, 2020, https://www.amchamchina.org/news/mofcom-vm-wang-shouwen-holds-back-to-work-call; and “European Chamber’s Conference Call with the Vice Minister of Commerce Wang Shouwen Joined by Multiple Departments on COVID-19’s Impact to FIEs,” European Chamber of Commerce in China, March 13, 2020, https://www.europeanchamber.com.cn/en/lobby-actions/3949/European_Chamber_s_Conference_Call_with_Vice_Minister_of_Commerce_Wang_Shouwen_Joined_by_Multiple_Departments_on_COVID_19_Impacts_to_FIEs.
105 Yukon Huang, “China’s Economic Growth Now Depends on the West,” Carnegie Endowment for International Peace, March 19, 2020, https://carnegieendowment.org/2020/03/19/china-s-economic-growth-now-depends-on-west-pub-81326.
Congressional Research Service
34
link to page 40 COVID-19: China Medical Supply Chains and Broader Trade Issues
was shuttered for much of February. This indicates that Chinese industry may have had sufficient stock already at ports for export when the crisis hit. This also signals the potential power of a resumed export push from China.75
China'106
China’s economic recovery is important to the United States and the global economy, as it is an important center of demand and supply. At the same time, during this period of global economic downturn, the United States and other countries are now potentially vulnerable to a concerted PRC export push and any effort it makes to take additional market share in strategic sectors.
Steel Overcapacity
Chinese overcapacity in steel has been highly contentious for its global impacts, and China could potentially see exports as a quick way to reduce inventories and secure needed cash. Similar to what happened during the global financial crisis in 2008-09, China is poised to take additional global market share in 2020 because it did not dial back production during the COVID-19 outbreak. Chinese blast furnaces continued to run during the COVID-19 crisis, and China'’s steel production for January-February 2020 was up 3% over the same period in 2019. Meanwhile, due to collapsing domestic demand and logistics constraints, China'’s finished steel inventories rose by 45% in January-February 2020 over the same period in 2019.76107 China'’s steel production at the end of 2019 was already at an all-time high of almost 1 billion tons, with China producing over 50% of global supply, according to the World Steel Association and China'’s State Statistical Bureau (Figure 6).108 China’s crude steel production recovered in July 2020, rising 9.1 percent year-on-year. China’s crude steel production during the January-July 2020 period is up 2.8% over the same period in 2019. In contrast, crude steel production over the same period is down 19.2% in the EU; down 18.7% in North America; down 24% in India; down 18.8% in Japan; and down 9.6% in South Korea.109
106 “China January-February Exports Tumble, Imports Down as Coronavirus Batters Trade and Business,” Reuters, March 6, 2020, https://www.reuters.com/article/us-china-economy-trade/china-january-february-exports-tumble-imports-down-as-coronavirus-batters-trade-and-business-idUSKBN20U05R.
107 Anindya Barman Zacks,” China Steel Output Rises Despite Oversupply and Coronavirus,” Yahoo Finance, March 19, 2020.
108 “World Steel in Figures 2019,” World Steel Association, https://www.worldsteel.org/media-centre/press-releases/2019/world-steel-in-figures-2019.html; Min Zhang and Tom Daly, “China 2019 Crude Steel Output Jumps 8.3%, Sets Second Straight Annual Record,” Reuters, January 16, 2020. 109 “July 2020 Crude Steel Production,” World Steel Association, August 24, 2020.
Congressional Research Service
35
link to page 41 link to page 41
COVID-19: China Medical Supply Chains and Broader Trade Issues
Figure 6(Figure 3).77
|
 |
Source: CRS with data from the World Steel Association. |
Export VAT Rebate
On March 17, 2020, China'’s Ministry of Finance announced it was increasing the export value added tax (VAT) rebate for almost 1,500 Chinese products, effective March 20, 2020. Most of the products (1,084) are receiving a 13% rebate; a small number (380) are receiving a 9% rebate.78 110 The export VAT rebate is a focused policy tool with quick effects that China typically employs to boost targeted exports during times of slowdown. It typically reduces the export VAT on products down to or close to zero. (See Table 5.)
(Table 7).
The rebates reflect a strong policy push for steel exports, as well as construction and building materials (e.g., insulation, wood products, glass and fiberglass). China is also promoting the export of a range of insecticides and industrial and organic chemicals. The rebates encourage the export of agricultural products in categories for which China promised to increase purchases from the United States—such as live breeding animals, meat and dairy—suggesting the government may be incentivizing exports for industries that might face additional U.S. imports. Absent in China'China’s policy push are incentives to encourage the sale of pharmaceuticals, PPE, and other medical products overseas.
The export VAT rebates also appear to be incentivizing China'appears to incentivize China’s export of wild animals and their byproducts overseas (Table 5)7). With assessments that COVID-19 could have originated in wild animals and potentially passed to humans in open air markets that sell these animals, China's ’s National People'’s Congress announced on February 24 a ban on the sale and consumption of wild animals in China.79111 While the export incentive might help the government to eradicate domestic markets by providing an economic incentive to export, this move could spread the risk to global markets.
Table 5. China'market.
110 “Announcement on Increasing the Export Tax Rebate Rate for Some Products,” PRC Ministry of Finance Announcement No. 15, March 17, 2020, http://www.chinatax.gov.cn/chinatax/n810341/n810755/c5146338/content.html. 111 “China’s Legislature Adopts Decision on Banning Illegal Trade, Consumption of Wildlife,” Xinhua, February 24, 2020, http://www.xinhuanet.com/english/2020-02/24/c_138814328.htm.
Congressional Research Service
36
link to page 42 link to page 42 link to page 42 COVID-19: China Medical Supply Chains and Broader Trade Issues
Table 7. China’s Export VAT Rebates, March 2020 (9%-13%)
Category
Examples
Livestock (Breeding)
s Export VAT Rebates, March 2020 (9%-13%)
|
Category |
Examples |
|
Livestock (Breeding) |
Horses, cattle, pigs, goats, sheep, chicken, turkey, |
ducks Meat (Fresh, Cold, Frozen, Byproducts) |
Beef, pork, chicken, lamb
Dairy
Milk and eggs
|
|
Dairy |
Milk and eggs |
Wild Exotic Animals (Live, Frozen, Horns, Claws, Fur, | Monkeys, edible snakes and reptiles, turtles, raptors, Feathers) ostrich, pigeon, beaver, civet; rhino horn |
Cotton, Flowers, Vegetables, Fruits, Oils, Nuts, Spices |
Orchids, garlic |
Orchids, garlic Industrial and Organic Chemicals; Insecticides |
Used in paints, nylon, latex, rubber, plastics, welding, anesthetics, and disinfectants
Dental and Paper Products
floss, paste, toilet paper, tissue, napkins, paper towels
Wood, Stone Mil s, Sandpaper
|
|
Dental and Paper Products |
floss, paste, toilet paper, tissue, napkins, paper towels |
|
Wood, Stone Mills, Sandpaper |
boxes, planks, windows, doors tables |
boxes, planks, windows, doors tables
Insulation and Drywall; Glass and Fiberglass
Pearls, Gemstones, Diamonds for Industrial Use
Steel and Nickel
| |
|
Pearls, Gemstones, Diamonds for Industrial Use |
|
|
Steel and Nickel |
117 products in Chapters 72 and 73 including bars and
rods, wire, strip, cold |
Source: China’s Ministry of Finance. China Pushing Ahead in Strategic Sectors
Now apparently past its peak of the COVID-19 outbreak, China is prepared to capitalize on the investments it made during the past few months to push ahead on goals outlined in its Made in China 2025 (MIC 2025) industrial plan, which includes several strategic health sectors (Figure 4)7). Introduced by China'’s State Council in May 2015, MIC 2025 is an ambitious state-led program that seeks to create competitive advantages for China in certain strategic industries. The plan aims to move China up the manufacturing value chain, expand its global market competitiveness, and reduce its reliance on foreign firms and their intellectual property (IP) over time. (See time (Figure 4)7). The program has been a major focus of the Trump Administration'’s Section 301 actions against China because of the distorting and predatory policies the initiative has set in motion related to technology transfer, intellectual property, and innovation.
Despite relying on China for certain PPE and API, the United States, together with Europe, is a global leader in high-end medical devices and novel pharmaceutical drug innovation, sectors in which China is seeking to gain ground through its industrial policies such as MIC2025. U.S. efforts to re-shore or diversify supply chains are primarily responding to crisis and may not be fully considering materiel that will be needed for vaccine deployment as well as strategic questions about how to sustain U.S. competitiveness in advanced medical sectors given China’s state-led policies that aim to dilute these advantages over time. Biotechnology, pharmaceuticals, and medical devices are key components of MIC 2025 industrial plans that support Chinese firms in efforts to increase their global market share of generic drugs and medical equipment, and develop new innovative drugs. Toward this end, the Chinese government restricts market access for foreign pharmaceutical firms. It requires foreign firms to conduct clinical trials in China, disclose proprietary information for drug trials and sales, and enter into partnerships to secure a spot on reimbursable drug lists. Moreover, medical equipment subsidies require that 60% of a product'product’s components be produced in China by a PRC firm.80113 These policies continue despite amendments to the Drug Administration Act in 2019 which were designed to make it easier for foreign pharmaceutical companies to operate in China.
China may have been serving its commercial ambitions in decisions it made during the height of the COVID-19 outbreak in China:
China has restricted access to medical information113 Robert D. Atkinson, “China’s Biopharmaceutical Strategy: Challenge or Complement to U.S. Industry Competitiveness?” ITIF, August 12, 2019, https://itif.org/publications/2019/08/12/chinas-biopharmaceutical-strategy-challenge-or-complement-us-industry. Congressional Research Service 38 COVID-19: China Medical Supply Chains and Broader Trade Issues China has restricted access to medical information and research about COVID-about COVID-19, including access for the U.S. Centers for Disease Control and Prevention (CDC), potentially putting U.S. science, research and development (R&D), and industry at a disadvantage.114 While some of these controls may be politically motivated, they also may be driven by China'’s market ambitions.The government'The government’s tight controls over biotechnology and pharmaceutical testing, treatment, and analysis in China could advantage its state firms.-
China also appears to see the race to develop a vaccine in terms of economic and
geopolitical competition. Since the COVID-19 outbreak, Chinese government-linked hackers have reportedly targeted several U.S. pharmaceutical companies developing COVID-19 vaccines and therapeutics, such as Moderna, and U.S. academic labs engaged in COVID-19 research, including the University of North Carolina.115 The Chinese government might prioritize access to any vaccine it develops according to both market access terms it negotiates as well as geopolitical priorities.116
China ordered that all viral samples from the beginning of the COVID-19
outbreak be destroyed or sent to the Wuhan Institute of Virology, a national lab run by China
'’s military. This move centralizes the government'’s knowledge about the potential origins of the virus and provides unique insights about its trajectory and treatment.81117 In March 2020, the Chinese Academy of Military Medical Sciences and CanSino Biologics, Ltd. were the first globally to begin a vaccine clinical study.118 The Wuhan Institute of Virology operates China'’s only biocontainment level 4 (P4) lab, a specialized facility for studies on highly contagious and fatal diseases. The Lab was developed by the Merieux Foundation under a government agreement between France and China.82 - 119
In another effort by the Chinese government to control access to important health
information, the World Health Organization (WHO)
'’s visit to China came over a month after the outbreak of the virus. Only a subset of the WHO-China Joint Mission on COVID-19 delegation was allowed to visit Wuhan.83 - 120 In July 2020,
114 Nectar Gan, Caitlin Hu and Ivan Watson, “Beijing Tightens Grip Over Coronavirus Research, Amid U.S.-China Row on Virus Origin,” CNN, April 16, 2020. 115 Christopher Bing and Marisa Taylor, “Exclusive: China-Backed Hackers ‘Targeted’ COVID-19 Vaccine From Moderna,” Reuters, July 30, 2020; and Julian E. Barnes and Michael Venutolo-Mantovani, “Race for Coronavirus Vaccine Pits Spy Against Spy,” The New York Times, September 5, 2020.
116 Chao Deng, “China Seeks to Use Access to Covid-19 Vaccines for Diplomacy,” The Wall Street Journal, August 17, 2020.
117 Gao Yu, Peng Yanfeng, Yang Rui, Feng Yuding, Ma Danmeng, Flynn Murphy, Han Wei, and Timmy Shen, “In Depth: How Early Signs of a SARS-Like Virus Were Spotted, Spread, and Throttled,” Caixin, February 29, 2020, https://www.caixinglobal.com/2020-02-29/in-depth-how-early-signs-of-a-sars-like-virus-were-spotted-spread-and-throttled-101521745.html; and Huang Shulun, Huang Huizhao, Peng Yanfeng, Liu Yuan, and Tang Ziyi, “Destroyed Market Samples Make it Impossible to Trace Origin of Deadly Virus, Experts Say,” Caixin, February 8, 2020, https://www.caixinglobal.com/2020-02-08/destroyed-market-samples-make-it-impossible-to-trace-origin-of-deadly-virus-expert-says-101513162.html.
118 Ryan Cross, “CanSino Publishes First COVID-19 Vaccine Data to Muted Response,” Chemical and Engineering News, May 28, 2020.
119 “China Inaugurates the First Biocontainment Level 4 Laboratory in Wuhan,” Wuhan Institute of Virology, China Academy of Sciences, February 3, 2015, http://english.whiov.cas.cn/News/Events/201502/t20150203_135923.html.
120 “Report of the WHO-China Joint Mission on Coronavirus 2019 (COVID-19), February 16-24, 2020, https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
Congressional Research Service
39
COVID-19: China Medical Supply Chains and Broader Trade Issues
the WHO team tasked with investigating the origins of COVID-19 met with officials in Beijing to draw up terms of reference and conditions of access with Chinese officials but has not yet traveled to Wuhan.121
China appears to have been slow to approve foreign drug patents potentially
China appears to have been slow to approve foreign drug patents potentiallyrelevant to COVID-19 until it needed them at the height of the crisis. For example, Gilead Sciences—a U.S. company based in California—had several patents for its antiviral drug Remdesivir'’s use in coronaviruses that have been pending approval since 2016. The Chinese government has been requiring the company to conduct clinical trials in China and did not approve these patents until well into the crisis. The Chinese government may have benefitted fromlong-standinglongstanding foreign patent application information that becomes public over time once a patent application is filed in China, even if the approval is still pending. The Chinese government also likely benefits from the insights gained through the clinical trials conducted in China and the viral samples that foreign companies share. Gilead, as well as other U.S. companies, sent the Chinese government samples of its drugs during the COVID-19 outbreak.84 - 122
The Chinese government cracked down on BrightGene BioMedical Technology
Co.—a PRC firm based in Suzhou, China—for the company
'’s premature announcement that it could compound a generic version of Remdesivir. Thegovernment'government’s move may be less of an effort to protect foreign firms than to position China'’s national labs. The Wuhan Institute of Virology, for example, has applied to patent an adaptation of Remdesivir.85123 This could potentially complicate Gilead'’s and other U.S. firms'’ way forward in China. -
China offered significant funding to Chinese biotech, pharmaceutical, and health
logistics companies to expand capacity and capabilities to combat COVID-19. For example, Jointown—a top Chinese medical supplier–issued preferential bonds in February 2020, and the State Council
'’s CITIC purchased private placement shares in the company.86
124
As the pandemic control measures continue in other countries, PRC official media is featuring stories about how the ChineseChina’s leadership is using its current control of medical production and supply chains to selectively help other countries, while promoting ties to China. State media is also highlighting China'’s interest in advancing its global medical leadership role. China'’s global health leadership was a key element of people-to-people exchanges envisioned in China'’s initial rollout of its "“One Belt One Road"” initiative in 2015.87125 During a call to Italian Prime Minister
121 Javier C. Hernadez and Amy Qin, “China Uses WHO Inquiry to Tout Coronavirus Response,” The New York Times, July 22, 2020.
122 Jay Barmann, “Bay Area-Based Gilead Sees Potential Legal Conflict with China Over its Coronavirus Drug,” SFIST, February 6, 2020, https://sfist.com/2020/02/06/bay-area-based-gilead-donates-experimental-anti-viral-drug-to-china/; and Elise Mak, “Gilead’s Remdesivir Enters China Phase III Trial to Fight Coronavirus,” BioWorld, February 3, 2020, https://www.bioworld.com/articles/432804-gileads-remdesivir-enters-china-phase-iii-trial-to-fight-coronavirus.
123 “Chinese Biotech Censured for False Claim on Gilead’s Virus Drug,” Bloomberg News, March 1, 2020, https://www.bloomberg.com/news/articles/2020-03-02/chinese-biotech-censured-for-false-claim-on-gilead-s-virus-drug; and Joe McDonald and Linda A. Johnson, “Chinese Scientists Ask for Patent on U.S. Drug to Fight Virus,” Associated Press, February 6, 2020, https://apnews.com/1fe943717b56b56cce5e733790f016dd.
124 Reuters, “Jointown Pharmaceutical To Issue Up To 1.5 Bln Yuan Worth of Renewable Bonds,” March 4, 2020. 125 “Vision and Actions on Jointly Building the Silk Road Economic Belt and 21stCentury Maritime Silk Road,” National Development and Reform Commission, Ministry of Foreign Affairs, and the Ministry of Commerce, March
Congressional Research Service
40
COVID-19: China Medical Supply Chains and Broader Trade Issues
During a call to Italian Prime Minister Conte on March 17, 2020, Chinese Communist Party Chairman Xi Jinping referenced a new Chinese government initiative—a Health Silk Road—that appears designed to promote Chinese leadership and products in the health sector.88126 Such efforts also aim to deflect criticism of China'China’s alleged corralling and destruction of the initial virus samples and efforts to prevent sharing of information among medical practitioners and the global community. Some experts have highlighted how this suppression of health information violates the obligations of WHO members to immediately share information about outbreaks for the safety of the world.89
127
The Chinese government reportedly undertook extraordinary measures during the COVID-19 outbreak to sustain R&D and manufacturing for priority national projects and in strategic sectors—such as telecommunications, microelectronics, and semiconductors—including in Wuhan, the epicenter of China'’s outbreak. These efforts have potential ramifications for U.S. and foreign firms'’ relative competitive market position as companies compete in 5G and other emerging sectors. This is particularly the case if their China operations were closed or are now significantly curtailed in the United States and other markets. According to the NikkeiNikkei Asia Review, in February and March 2020, the Chinese government operated special transportation and quarantined dormitories at Yangtze Memory Technology, Co., Ltd. (YMTC), China'’s national champion to develop memory chips. YMTC is located in eastern Wuhan. The government saw continued operations as an issue of national security and issued special local and central government dispensation to keep the facility operational amidst the outbreak.90128 Separate reports indicate that HiSilicon—the semiconductor subsidiary of China'’s leading telecommunications equipment company Huawei—also sustained operations during the outbreak.91 Huawei's chairman and chief executive told The Wall Street Journal on March 25, 2020 that the company plans to boost its research and development budget in 2020 by $5.8 billion to more than $20 billion.92
129 Issues for Congress
Congress faces choices in the near-term that will affect not only the immediate situation, but also the longer-range U.S. trade and economic trajectory vis-a-vis China, with a potentially significant impact on the global economy as well. The outbreak of COVID-19 has prompted a sharp collapse of transportation, services, and manufacturing production—including supply shortages of essential medical and health care products needed to contain COVID-19. The COVID-19 pandemic has also precipitated a sharp downturn in consumer demand, first in China and now globally. Questions already brewing since the imposition of U.S. Section 301 tariffs are intensifying congressional concerns and debates about potential short-term and long-term steps to address U.S. supply chain dependence on China for critical products, and the potential ramifications of these dependencies. These ramifications could be particularly marked in times of crisis or of PRC nationalization of industry. At the same time, some U.S. companies and
28, 2015, https://reconasia-production.s3.amazonaws.com/media/filer_public/e0/22/e0228017-7463-46fc-9094-0465a6f1ca23/vision_and_actions_on_jointly_building_silk_road_economic_belt_and_21st-century_maritime_silk_road.pdf.
126 Li Yan, “Xi Says China to Send More Medical Experts to Italy,” Xinhua, March 17, 2020, http://www.ecns.cn/m/news/politics/2020-03-17/detail-ifzunmih1236562.shtml.
127 James Kraska, “China is Legally Responsible for COVID-19 Damage and Claims Could be in the Trillions,” War on the Rocks, March 23, 2020, https://warontherocks.com/2020/03/china-is-legally-responsible-for-covid-19-damage-and-claims-could-be-in-the-trillions/.
128 Cheng Ting-Fang and Lauly Li, “China Lets Wuhan Tech Plants Bypass Lockdown to Stay Open,” Nikkei Asian Review, March 4, 2020, https://asia.nikkei.com/Spotlight/Coronavirus/China-lets-Wuhan-tech-plants-bypass-lockdown-to-stay-open.
129 “Huawei, Chinese Chip Makers Keep Factories Humming, Despite the Virus,” Reuters, February 2, 2020, https://www.reuters.com/article/us-china-health-tech-huawei/huawei-chinese-chip-makers-keep-factories-humming-despite-virus-outbreak-idUSKBN1ZX0CX.
Congressional Research Service
41
COVID-19: China Medical Supply Chains and Broader Trade Issues
crisis or of PRC nationalization of industry. At the same time, some U.S. companies and Members of Congress are calling for lowering tariffs on goods from China. The urgent need for pharmaceutical and medical supplies is fueling systemic market pressures to increase U.S. reliance on China trade because China is an important source of many of these critical inputs and products. Whether and on what terms the Chinese government might be willing to export medical supplies to the United States remains uncertain.
Dependency of U.S. Health Care Supply Chains on China
The currentexperience of shortages of critical medical supplies in the United States has exposed current U.S. health care dependencies on China. As China positions its industries to realize its MIC 2025 goals in biotechnology, pharmaceuticals, and medical equipment, the Chinese government is pursuing industrial polices to advance into higher positions in the global industrial value chain, raising longer-range questions about what this might portend for U.S. reliance on China as an increasingly competitive supplier.
As China'’s manufacturing capacity comes back online while the United States and other major global markets continue to grapple with COVID-19, the Chinese government appears to be selectively releasing some medical supplies for overseas delivery. China appears to be selecting designated countries, at least to some extent (although the precise degree cannot be determined), according to political calculations and has been playing up its role in Chinese state propaganda, as evidenced with China'’s deliveries to Italy and Serbia.93130 Most foreign governments appear to be paying for these supplies although a small subset of packages may be aid. There are also reports by other countries that some of China'’s medical supplies and testing kits are faulty.94131 In a sign that China might be using the crisis to push substandard products or gain market share in developed markets over traditional U.S. suppliers based in China that produce for export, PRC state propaganda has blamed shortages in March 2020 on alleged FDA failures to certify Chinese products for import.95132 This raises the question of why products made by U.S. firms in China that are already FDA certified arewere not first in line for export to the United States given that these firms also expanded capacity during the crisis in China. Several prominent U.S. companies, including 3M, have indicated they do indicated in the early stages of the United States’ COVID-19 outbreak that they did not have PRC government authorization to export.96
133
In this environment, Congress faces choices about how best to incentivize production of health supplies in the United States, potentially in collaboration with other countries, to counter COVID-19 and future pandemics, and/or whether to impose any conditions on this production. In addition to a focus on lower-value items such as PPE, Congress might focus on ways to sustain U.S. industrial leadership in advanced medical equipment and pharmaceutical innovation. With an eye to China'’s industrial policies, Congress may also consider the potential longer-term advantages and disadvantages of diversifying U.S. supply and on-shoring of certain capabilities. Congress
130 Li Yan, “Xi Says China to Send More Medical Experts to Italy,” Xinhua, March 17, 2020, http://www.ecns.cn/m/news/politics/2020-03-17/detail-ifzunmih1236562.shtml934157.shtml; Reuters, China sends Serbia help to halt coronavirus spreading,” March 21, 2020. 131 Wendy Wu, “Coronavirus: Don’t Politicize Medical Supply Problems, China Says,” South China Morning Post, March 30, 2020, https://www.scmp.com/news/china/diplomacy/article/3077537/coronavirus-chinas-ambassador-closely-following-netherlands.
132 “Barriers Should be Removed to Send Medical Supplies to US,” Global Times, March 24, 2020, https://www.globaltimes.cn/content/1183647.shtml.
133 Keith Bradsher and Liz Alderman, “The World Needs Masks. China Makes Them—But Has Been Hoarding Them,” The New York Times, March 16, 2020, https://www.nytimes.com/2020/03/13/business/masks-china-coronavirus.html.
Congressional Research Service
42
COVID-19: China Medical Supply Chains and Broader Trade Issues
and disadvantages of diversifying U.S. supply and on-shoring of certain capabilities. Congress may also want to consider potential collaboration with like-minded countries, and ways to counter the effects on lesser-developed economies that could be hit particularly hard by COVID-19. China is likely to seek to retain the medical market share and edge it gains through COVID-19, particularly as these gains help advance China'’s MIC 2025 industrial policy goals in biotechnology, pharmaceuticals, and medical equipment. At the same time, the United States and other countries may seek to diversify away from China because of vulnerabilities highlighted during the outbreak.
Nascent proposals to diversify away from China concerns and risks—such as UK Prime Minister Boris Johnson’s suggestion of a D-10 alliance and the U.S. government’s Clean Network strategy—advocate for closer trading ties among like-minded countries in sensitive technologies and sectors.134 Recent legislative action related to these issues includes:
-
P.L. 116-136, The Coronavirus Aid, Relief, and Economic Security (CARES) Act
includes several provisions that expand drug shortage reporting requirements to include APIs and medical devices. The bill also requires certain drug manufacturers to draw up risk management plans and requires the FDA to maintain a public list of medical devices that are determined to be in shortage. Additionally, the bill directs the National Academies of Science, Engineering, and Medicine to conduct a study of pharmaceutical supply chain security. The CARES Act also waives certain congressional oversight and reporting requirements under the Defense Production Act of 1950
'’s (DPA; 50 U.S.C. §§4501 et seq.) Title III Expansion of Productive Capacity and Supply, which governs purchases and loans made by the federal government to expand productive capacity in promotion of national defense, broadly defined. H.R. 8406, The Heroes Act, would introduce additional reporting requirements for drug manufacturers that obligate them to report drugs that are vulnerable to supply chain risks that could lead to shortages, and would introduce new penalties for failing to report. The bill would also introduce measures to strengthen U.S. competitiveness in advanced pharmaceutical manufacturing by enhancing the advanced manufacturing programs of the FDA and creating a new supply chain flexibility manufacturing pilot program. The bill would also encourage more research into ways to enhance domestic production of critical APIs and certain finished dosage drugs by designating certain research universities as “National Centers of Excellence in Continuous Pharmaceutical Manufacturing.” The Heroes Act was originally introduced as H.R. 6800 and was passed by the House on May 15, 2020; an updated version of the bill (H.R. 8406) was introduced on September 29, 2020. S. 4049, The National Defense Authorization Act for Fiscal Year 2021 as passed by the Senate, contains a provision directing the Office of the Director of National Intelligence (ODNI) to submit a report to Congress assessing, among other risks, the risk of the Chinese government restricting or manipulating global medical and pharmaceutical supply chains to advance economic and political objectives. H.R. 6395, the National Defense Authorization Act for Fiscal Year 2021 as passed by the House, contains a provision tasking HHS with preparing a report that contains a list of the drugs and vaccines on the joint deployment formulary, identifies each drugs APIs and country of origin, and provides an 134 Lucy Fisher, “Downing Street Plans New 5G Club of Democracies,” The Times, May 29, 2020 and Aaron Friedburg, “The United States Needs to Reshape Global Supply Chains,” Foreign Policy, May 8, 2020, and “The Clean Network Safeguards America’s Assets,” U.S. Department of State Fact Sheet, August 11, 2020. Congressional Research Service 43 COVID-19: China Medical Supply Chains and Broader Trade Issues additional list of drug manufacturers in the United States owned in whole or in part by a foreign entity. H.R. 7856, The Intelligence Authorization Act for Fiscal Year 2021, would also require ODNI to submit an additional report detailing China’s regulatory practices governing pharmaceutical and PPE manufacturing, as well as an estimate of the total percentage of APIs produced globally that originate in China. S. 4324, The Restoring Critical Supply Chains and Intellectual Property Act, contains multiple provisions that aim to re-shore supply chains across a range of sectors critical to public health and national security. Measures in the bill include a 30% investment tax credit for qualified manufacturers of medical PPE, stricter domestic purchasing requirements for the Strategic National Stockpile maintained by HHS, a new grant program to incentivize U.S. domestic production of advanced semiconductor chips, and funding for a new program to identify critical mineral supplies and address gaps in critical mineral supply chains. S. 3538 and H.R. 6393, The Strengthening America's Supply Chain and National Security Act, would require companies to report on the sources of their APIs and would tighten laws encouraging the U.S. Department of Veterans Affairs to buy American pharmaceuticals. The bill calls for federal financing guarantees to U.S. medical supply companies with production in the United States and would increase the tax deduction temporarily for businesses investing in medical equipment and facilities related to COVID-19. S. 3343 and H.R. 6049, The Medical Supply Chain Security Act, calls for enhanced security of the medical supply chain and enhanced FDA authority to request information about the sources of drugs and medical devices. It would require medical device manufacturers to report expected shortages to the FDA. S. 3635 and H.R. 6482, The Protecting Our Pharmaceutical Supply Chain from China Act of 2020, would require the FDA to establish a registry to track APIs and institute a country-of-origin label for imported drugs. The bill would provide economic incentives for producing pharmaceuticals and medical equipment in the United States. The bill also would prohibit federal agencies and health facilities from purchasing APIs and other pharmaceutical products manufactured in China without an FDA waiver certifying that China is the sole source.135 H.R. 6731, The Securing America’s Pharmaceutical Supply Chain Act, would require executive agencies to restrict purchases of pharmaceuticals to drugs “over 50 percent sourced, manufactured, and assembled in the United States.” The bill would also direct USTR to modify U.S. product coverage under all free trade agreements and the WTO Government Procurement Agreement (GPA) to exclude coverage of essential medicines and certain medical products. H.R. 5982, The Safe Medicine Act, would direct HHS to assess vulnerabilities in the U.S. pharmaceutical supply chain by issuing a report that examines U.S. dependence on China for critical APIs and gaps in domestic pharmaceutical manufacturing capabilities. 135 An earlier version of S. 3635 was introduced in the 116th Congress as S. 3537. Congressional Research Service 44 COVID-19: China Medical Supply Chains and Broader Trade Issues H.R. 6386, The No Chinese Handouts In National Assistance (CHINA) Act, would prohibit any funds made available in Appropriations acts for FY2020 from being used to compensate any individual or business controlled by the Chinese government. The Act adopts the definition of government control established in Section 721(a) of the Defense Production Act of 1950 (50 U.S.C. §4565(a)). H.R. 4710, The Pharmaceutical Independence Long-Term Readiness Reform Act, would direct the Department of Defense to include a section in each national defense strategy that outlines steps to address gaps in the U.S. pharmaceutical manufacturing base and strengthen pharmaceutical supply chains with single points of failure. Several Members of Congress also have introduced bills to amend certain provisions under the Defense Production Act of 1950 (DPA; 50 U.S.C. §§4501 et seq.) and ease its implementation. S. 4050, the Public Health Emergency Production Act of 2020 (PHEPA) would create an office in HHS with responsibility for a variety of DPA responsibilities, including a freestanding DPA Title III office, which would be led by an official at the rank of Deputy Assistant Secretary. Some Members have also introduced several resolutions in the House and Senate that call on the President to use DPA authorities to facilitate the production of medical supplies. H.R. 6395, the National Defense Authorization Act for Fiscal Year 2021 as passed by the House, for example, contains provisions that would expand the definition of health resources critical to national security to include PPE and COVID-19 testing equipment. S. 4339 and H.R. 7836, the Masks for All Act of 2020, would direct FEMA to, among other things, use the DPA to provide for the manufacture and distribution of face masks for every individual in the United States in response to the COVID-19 pandemic. Despite recent legislation to spur PPE production and concerns by expressed by Members of Congress over the supply of available PPE, the Administration has not consistently used DPA authorities to expedite PPE contracts.136 In an effort to address the ambiguity of the Administration’s response, several Members of Congress have introduced legislation such as H.R. 6390 and S. 3568 that would require the President to use authorities under the DPA to require emergency production of medical equipment to respond to the COVID-19 outbreak. In addition to recent legislation introduced by Members of Congress, the Trump Administration drafted an Executive Order in mid-March 2020 that seeks to increase U.S. production capacity while eliminating loopholes that have allowed the U.S. government to buy pharmaceuticals, PPE, and ventilators from overseas. 137 Released on August 6, 2020, this Executive Order 13944 mandates that federal agencies “conduct the procurement of Essential Medicines, Medical Countermeasures, and Critical Inputs by: using procedures to limit competition to only those Essential Medicines, Medical Countermeasures, and Critical Inputs that are produced in the United States; and dividing procurement requirements among two or more manufacturers located in the United States, as appropriate." Additionally, the order requires USTR to “take all appropriate action to modify United States federal procurement product coverage” under the WTO Agreement on Government Procurement and other relevant trade agreements. The Executive Order also calls for the HHS Secretary to identify U.S. supply chain vulnerabilities for essential medicines, medical capabilities, and critical inputs within 180 days of the issuance of the 136 For recent developments in the use of DPA to procure medical supplies, see CRS Insight IN11470, Defense Production Act (DPA): Recent Developments in Response to COVID-19, by Michael H. Cecire and Heidi M. Peters; for an in-depth discussion of DPA history and authorities, see CRS Report R43767, The Defense Production Act of 1950: History, Authorities, and Considerations for Congress, by Michael H. Cecire and Heidi M. Peters. 137 “White House Working on Order to Cut U.S. Dependency on Foreign Medicines,” Reuters, March 16, 2020. Congressional Research Service 45 COVID-19: China Medical Supply Chains and Broader Trade Issues Executive Order. HHS is called to streamline requirements and accelerate certain applications. The Executive Order also calls for federal agencies to provide detailed procurement details for the prior three years by no later than December 31, 2021, and annually thereafter.138 The Administration also took steps to increase funding for U.S. API production capacity for critical medicines. These steps included awarding a $354 million contract to a corporation in Richmond, Virginia, and a $765 million loan to Eastman Kodak to develop API production capacity.139 Other U.S. Supply Chain Dependencies COVID-19 provides a direct learning experience about the direct effects and costs of a serious disruption or cutoff of critical supplies from China to the United States. Key broader questions facing the United States that have serious implications for future economic and trade relations include: What are the consequences for U.S. interests when China nationalizes production and distribution and hardens its borders as it did during the COVID-19 crisis? What happens if Chinese government planners corner global supply alternatives? What happens if the United States hardens its own borders? What happens if U.S. allies and partners are in crisis and turn to national tools and approaches? What supply lines are available to the United States? What is current baseline U.S. production capacity and what is U.S. production capacity in the event an Administration invokes the Defense Production Act (DPA)? What control do chief executive officers of U.S. companies or the U.S. government have over U.S. corporate facilities and operations that are nationalized in China? What are U.S. dependencies on China in other critical areas such as microelectronics? U.S. Market Competitiveness and Tariff Policy Congress faces a series of interrelated questions about whether and how to calibrate trade policy to best position the United States in the current crisis and beyond. In response to a U.S. investigation of China’s unfair trading practices under Section 301, since 2018, the United States has imposed a series of tariffs and China has responded with a series of counter tariffs that now affect a majority of trade between the two countries. Temporary tariff relief for medical supplies and pharmaceuticals could incentivize imports for the United States and other markets, but tariff policy cannot address the deeper issues of supply shortages, export constraints imposed by a number of countries including China, and product certification requirements in the United States 138 Executive Order 13944, “Combating Public Health Emergencies and Strengthening National Security by Ensuring Essential Medicines, Medical Countermeasures, and Critical Inputs Are Made in the United States,” 85 Federal Register 49929, August 6, 2020. 139 U.S. International Development Finance Corporation, “DFC to Sign Letter of Interest for Investment in Kodak’s Expansion Into Pharmaceuticals,” press release, July 28, 2020; U.S. Department of Health and Human Services, “HHS, Industry Partners Expand U.S.-Based Pharmaceutical Manufacturing for COVID-19 Response,” press release, May 19, 2020. Congressional Research Service 46 COVID-19: China Medical Supply Chains and Broader Trade Issues and other markets. Tariff liberalization has been insufficient to address industrial policies within borders such as regulatory standards, procurement terms, and local content requirements that China and others impose in a range of sectors including pharmaceuticals and medical equipment.140 Actions by countries around the world to impose export barriers during the pandemic highlight potential gaps and limits to the power of WTO rules prohibiting export bans during times of global crisis. These actions also raise questions about what new rules or protocols might be needed in the future.141 Liberalization of U.S. import requirements may have also created some of the challenges the United States is facing now, such as loosening the requirements for U.S. pharmaceutical firms to report on shortages and how they classify imported content for finished products that qualify as U.S. products. The potential for China to overwhelm global markets as it leans on exports for economic recovery raise questions about whether additional policy measures might be needed. Rather than waiting until market injury has already occurred to seek damages, for example, Congress may want to be watching trade patterns for signs of import surges and oversee the Administration’s potential use of safeguard measures. Similar to the Australian government’s decision on March 29, 2020 to impose new temporary restrictions on all foreign investment proposals out of concern that strategic investors—particularly those of Chinese origin—might target distressed assets, Congress may want to carefully monitor these trends. Information and Data Gaps The outbreak of COVID-19 has exposed gaps in U.S. understanding of U.S. domestic competencies and dependencies on China and other sources of global supply. Vulnerabilities regarding raw materials, such as APIs, are not well recorded in trade and industry data. They are particularly complicated to track when materials are shipped from China and processed in a third market such as India. In similar fashion, the United States has relaxed definitions of what qualifies as a U.S. product with imported content, masking the extent to which domestically produced products may still rely on inputs from overseas. Pharmaceutical company stockpiles are proprietary, and companies do not have to report on reserves. They are only required to report when they have a shortfall, which does not leave enough time, particularly in times of emergency, for national and contingency planning. Under the International Investment Survey Act of 1976 (22 U.S.C. §3101 et seq.), the President has wide authority over the collection of corporate activity abroad for statistical and analytic purposes. The Act also confers on the President the authority to request mandatory surveys of companies under specific deadlines with the ability to invoke civil and criminal penalties for noncompliance. The President has the authority to study the adequacy of current information and recommend improvements, and the Act requires him to report to Congress.142 140 Jennifer A. Hillman, “Six Proactive Steps in a Smart Trade Approach to Fighting COVID-19,” ThinkGlobalHealth, March 20, 2020, https://www.thinkglobalhealth.org/article/six-proactive-steps-smart-trade-approach-fighting-covid-19 Anabel Gonzalez, “A Memo to Trade Ministers on How Trade Policy Can Fight COVID-19,” PIEE, March 23, 2020, https://www.piie.com/blogs/trade-and-investment-policy-watch/memo-trade-ministers-how-trade-policy-can-help-fight-covid. 141 See CRS In Focus IF11551, Export Restrictions in Response to the COVID-19 Pandemic, by Christopher A. Casey and Cathleen D. Cimino-Isaacs. 142 https://uscode.house.gov/view.xhtml?path=/prelim@title22/chapter46&edition=prelim; and “Legal Authority and Confidentiality of International Survey Collections,” U.S. Bureau of Economic Analysis, https://www.bea.gov/about/legal-authority-and-confidentiality-international-survey-collections. Congressional Research Service 47 COVID-19: China Medical Supply Chains and Broader Trade Issues To address these issues, Congress could consider whether to request the President to invoke his authority over the U.S. government’s collection of data on corporate activity abroad. These corporate surveys could obtain specific supply chain information about the status of PPE and medical supply production, distribution, and export policy situation facing U.S. companies overseas, including in China. The surveys also could cover other sectors of potential congressional concern. This information could inform legislation that Congress has already passed or is considering with regard to overseas supply chains, including sourcing from China. The COVID-19 pandemic has highlighted potential limitations in the U.S. medical product supply chain, including concerns that many raise about U.S. reliance on foreign manufacturers and a lack of transparency and diversification in key areas. Some Members of Congress have raised concerns regarding gaps in U.S. Government’s understanding of U.S. domestic competencies and dependencies on China and other sources of global supply.143 Possible vulnerabilities regarding raw materials and inputs, such as active pharmaceutical ingredients (APIs), are not well recorded in official trade and domestic industry data. They might be particularly difficult to track if they originate in one country but are subsequently processed in another. While facilities that manufacture drugs and medical devices for the U.S. market generally are required to register with FDA, the agency has limited visibility into the quantity produced at a specific facility, particularly with respect to raw materials or APIs. In addition, the United States has relaxed definitions of what qualifies as a U.S. product with imported content, which may mask the extent to which domestically produced products rely on foreign inputs.144 In response to these concerns, Congress has considered legislation to help regulators, stakeholders, and the public better understand the medical product supply chain. The Coronavirus Aid, Relief, and Economic Security Act (CARES Act; P.L. 116-136), for example, requires HHS to contract with the National Academies of Science, Engineering, and Medicine (NASEM) to examine and report on the security of the U.S. medical product supply chain, including U.S. dependence on critical drugs and devices (e.g., medical PPE) from other countries.145 The CARES Act also included a provision that aims to address some of these gaps by requiring registered drug and API producers to report to the FDA, on an annual basis, the amount of drugs manufactured for domestic distribution. Legislation has been introduced that would expand this requirement to medical devices (e.g., PPE) and would make the requirements quarterly.146 The CARES Act also provided FDA the explicit authority to require certain device manufacturers to report interruptions or discontinuances in manufacturing during or prior to a public health emergency; to take certain actions to mitigate shortages; and to make public a list of devices that are in shortage.147 Congress could consider expanding reporting requirements in future legislation to include requiring manufacturers of all medical devices to report to FDA actual or forecasted increases in demand that may lead to a shortage, or to report actions taken by other regulatory 143 U.S. Congress, Senate Committee on Homeland Security and Governmental Affairs, Evaluating the Federal Government’s Procurement and Distribution Strategies in Response to the COVID-19 Pandemic, hearing, 116th Cong., 2nd sess., June 9, 2020. 144 The lack of statutory definitions of various terms (e.g., “manufactured” in the United States) may yield different determinations for the same product. Moreover, the “substantial transformation” test used by U.S. Customs and Border Protection (CBP) to determine a product’s country of origin for trade purposes is complex, fact-specific, and thus inherently subjective in nature. 145 P.L. 116-136, §3101. 146 See, for example, S. 3781 (116th Congress). 147 FFDCA §506J, as established by P.L. 116-136, §3121. Congressional Research Service 48 COVID-19: China Medical Supply Chains and Broader Trade Issues authorities that could affect U.S supply (e.g., export restrictions). This may help FDA better anticipate and take steps to prevent shortages. While medical products manufacturers are required to report various supply chain information to FDA, this information may not be shared with other agencies or departments. As such, legislation has been introduced in the 116th Congress that would require FDA to share certain supply chain information with the ASPR and DOD.148 Legislation also has been introduced that would require the Secretaries of HHS, Homeland Security, and Defense to individually conduct annual risk assessments of the medical product supply chain and submit those assessments to Congress.149 This information could be used to guide PPE production and acquisition efforts. Some Members have also introduced legislation that would require the U.S. government to review and report on the origin of pharmaceuticals sold in the United States and the role that foreign manufacturing plays in medical supply chains.150 Other congressional proposals would require drug makers to report on reserves, since requiring them to do so only when they have shortages that may not leave enough time, particularly in times of emergency, for national and contingency planning. While there may not be a single legislative solution to measure and manage supply chain dependencies and risks, Congress could consider authorizing federal agencies to collect more data on individual firms’ activities in the United States and abroad. In the past, Congress has taken similar steps to monitor U.S. investment abroad and foreign investment in the United States (see 22 U.S.C. §§ 3101-3108). Agencies could obtain, analyze, and report specific supply chain information about the status of PPE and medical goods production and distribution without disclosing business confidential information that could seriously prejudice firms’ interests. Surveys also could help assess the overall production capabilities of U.S.-based producers in industries or sectors of congressional concern. Alternatively, Congress could direct some agencies to collect data on federally owned public and defense stockpiles of certain items. While this would be a more targeted effort, it might be easier to manage and provide comprehensive data far more quickly and at less expense to the government. This information could inform legislation that Congress has already passed or is considering with regard to domestic production and overseas supply chains, including sourcing from China. Unique Role of the U.S. Federal Government At a time when U.S. health care systems, states, and countries overseas are seeking to secure limited medical supplies, the U.S. federal government has a unique role to play in ensuring adequate domestic and global production, contracting of supply (both domestically and globally), and distribution of these resources. Even as new capacity might be available in China, for example, who are the U.S. actors positioned to try to secure this supply and through what pathways? Lack of coordination at the federal level has led states to scramble and compete against each other for critical medical supplies in the current crisis. Among the key questions related to these issues, Congress may explore answers to such questions as: How does the U.S. federal government position itself vis-a-vis U.S. state and private actors? 148 See, for example, S. 3781 (116th Congress). 149 See, for example, S. 3780 (116th Congress). 150 See, for example, S. 3537 (116th Congress). Congressional Research Service 49 COVID-19: China Medical Supply Chains and Broader Trade Issues How does the U.S. federal government position itself vis-a-vis other foreign governments trying to secure similar supplies? What is the U.S. government’s posture toward supplies needed in the developing world? How might expanded production capacity created in the United States not only help the U.S. market but also those of other countries, in the near term and over the longer term? U.S. Leadership on Global Medical Trade The current COVID-19 pandemic provides a unique opportunity to reaffirm U.S. global leadership on trade and health issues and to counter China’s nationalization and likely politicization of its domestic medical supply production capacity. China’s export restraints and cornering of the global supply of medical products ahead of others in February 2020 have created strains on the open trade system, further incentivizing other countries to close borders and restrict any access to supplies they may have. These moves also have given China market power over other countries’ procurement decisions as governments around the world grapple with how best to secure critical supplies. Early signs show that China is closely controlling and releasing supplies to other governments through contracts and some aid in ways that seek to improve China’s global image and may come with other quid pro quo terms that are not yet visible. China’s economic recovery ahead of others could further challenge and undermine key tenets of the open trade system, particularly if China exports pent up domestic capacity with a disregard for what the current state of the global economy is prepared to absorb on market terms. While some European countries have imposed export restraints on their health supplies, some politicians in Europe are concerned about how the Chinese government is manipulating the crisis and China’s position in global supply chains for political gain.151 Some analysts have expressed concern that China is trying to position itself as a responsible global leader in health, while violating the core tenets of WHO membership in failing to share critical information and access in the critical first few weeks as the crisis emerged in Wuhan. Members concerned about maintaining U.S. global economic leadership during the COVID-19 pandemic may consider using hearings, legislation, and statements to communicate key issues to be addressed. Possible questions for Congress in the context of COVID-19 include: whether to prioritize economic openness and free flows of information; whether to prioritize diversifying sources of medical supplies, and if so, how; how best to overcome current and future bottlenecks in health care supply chains in the United States and partner nations; whether to respond to China’s attempts to control the global narrative about key COVID-19 events, and if so, how; and whether to look to reform global health and trade governance in light of COVID- 19 developments, and if so, how. Some Members have questioned the role of China and the WHO during the initial COVID-19 outbreak and are raising questions about the need to reform global health governance. Other 151 “The Coronavirus and the New World it is Creating,” European Union External Action Office, March 23, 2020, https://eeas.europa.eu/headquarters/headquarters-homepage_en/76379/The%20Coronavirus%20pandemic%20and%20the%20new%20world%20it%20is%20creating. Congressional Research Service 50 COVID-19: China Medical Supply Chains and Broader Trade Issues Members have looked to set a clear chronology of events in the COVID-19 outbreak to maintain an accurate record that is not distorted by Chinese state propaganda. Some Members are also looking at the social media platforms that the Chinese government is using to convey state propaganda—such as Twitter—and raising questions about whether this access should be allowed. Several Members have expressed an interest in potential measures to hold China accountable for its slowness to acknowledge, address, and share information regarding the outbreak of COVID-19 as required by WHO members. Congressional Research Service 51 COVID-19: China Medical Supply Chains and Broader Trade Issues Appendix A. U.S. Imports of Select Medical Products Table A-1. U.S. Imports of Pharmaceuticals and Medical Equipment, Products, and Supplies in 2019 Economy Value (US$) Ireland 35,797,919,666 Germany 25,416,992,979 China 20,744,036,029 Switzerland 19,115,982,191 Mexico 15,758,366,376 Italy 9,356,424,042 Canada 9,072,982,790 India 8,325,151,620 Japan 8,126,636,035 Singapore 7,947,308,765 Rest of the World 64,911,471,228 Total $224,573,271,721 Source: CRS using the World Customs Organization’s “HS Classification Reference for COVID-19 Medical Supplies;” Gary Clyde and Jeffrey J. Schott’s “List of Pharmaceutical and Medical Device Products by Harmonized System (HS) Code” in Local Content Requirements: A Global Problem; and Chad Bown’s “Trump’s Trade Policy Is Hampering the U.S. Fight Against COVID-19.” Data sourced from the U.S. International Trade Commission’s DataWeb and Global Trade Atlas. Notes: The figures presented here cover product categories at the HTS six-digit level. Author Information Karen M. Sutter, Coordinator Michael D. Sutherland Specialist in Asian Trade and Finance Analyst in International Trade and Finance Andres B. Schwarzenberg Analyst in International Trade and Finance Congressional Research Service 52 COVID-19: China Medical Supply Chains and Broader Trade Issues Disclaimer This document was prepared by the Congressional Research Service (CRS). CRS serves as nonpartisan shared staff to congressional committees and Members of Congress. It operates solely at the behest of and under the direction of Congress. Information in a CRS Report should not be relied upon for purposes other than public understanding of information that has been provided by CRS to Members of Congress in connection with CRS’s institutional role. CRS Reports, as a work of the United States Government, are not subject to copyright protection in the United States. Any CRS Report may be reproduced and distributed in its entirety without permission from CRS. However, as a CRS Report may include copyrighted images or material from a third party, you may need to obtain the permission of the copyright holder if you wish to copy or otherwise use copyrighted material. Congressional Research Service R46304 · VERSION 3 · UPDATED 53productive capacity in promotion of national defense, broadly defined. - S. 3538 would require companies to report on the sources of their APIs and would tighten laws encouraging the U.S. Department of Veteran Affairs to buy American pharmaceuticals. The bill calls for federal financing guarantees to U.S. medical supply companies with production in the United States and would increase the tax deduction temporarily for businesses investing in medical equipment and facilities related to COVID-19.97
- S. 3343, The Medical Supply Chain Security Act, calls for enhanced security of the medical supply chain and enhanced FDA authority to request information about the sources of drugs and medical devices. It would require medical device manufacturers to report expected shortages to the FDA. A companion bill, H.R. 6049, was introduced in the House of Representatives on March 2, 2020.
- S. 3537 would require the FDA to establish a registry to track APIs and institute a country-of-origin label for imported drugs. The bill would provide economic incentives for producing pharmaceuticals and medical equipment in the United States. The bill also would prohibit federal agencies and health facilities from purchasing APIs and other pharmaceutical products manufactured in China without an FDA waiver certifying that China is the sole source.98
- H.R. 5982, The Safe Medicine Act, would direct HHS to assess vulnerabilities in the U.S. pharmaceutical supply chain by issuing a report that examines U.S. dependence on China for critical APIs and gaps in domestic pharmaceutical manufacturing capabilities.
- H.R. 6386, The No Chinese Handouts In National Assistance (CHINA) Act, would prohibit any funds made available in Appropriations acts for FY2020 from being used to compensate any individual or business controlled by the Chinese government. The Act adopts the definition of government control established in Section 721(a) of the Defense Production Act of 1950 (U.S.C. 4565(a)).99
- H.R. 4710, The Pharmaceutical Independence Long-Term Readiness Act, would direct the Department of Defense to include a section in each national defense strategy that outlines steps to address gaps in the U.S. pharmaceutical manufacturing base and strengthen pharmaceutical supply chains with single points of failure.
- S. 3432, The Securing America's Medicine Cabinet Act of 2020, would take steps to strengthen U.S. competitiveness in advanced pharmaceutical manufacturing by enhancing the advanced manufacturing programs of the FDA. It also would designate certain research universities as "National Centers of Excellence in Advanced Pharmaceutical Manufacturing."
Several Members of Congress have introduced bills to amend certain provisions under the Defense Production Act of 1950 (DPA; 50 U.S.C. §§4501 et seq.). Some Members have also introduced several resolutions in the House and Senate that call on the President to use DPA authorities to facilitate the production of medical supplies. Bills and resolutions related to DPA are compiled and summarized inAppendix B.
In addition to recent legislation introduced by Members of Congress, the Trump Administration reportedly drafted an Executive Order in mid-March 2020 that seeks to increase U.S. production capacity while eliminating loopholes that have allowed the U.S. government to buy pharmaceuticals, PPE, and ventilators from overseas.100
Other U.S. Supply Chain Dependencies
COVID-19 provides a direct learning experience—potentially more compelling than any war game or natural disaster simulation—about the direct effects and costs of a serious disruption or cutoff of critical supplies from China to the United States. Key broader questions facing the United States that have serious implications for future economic and trade relations include:
- What are the consequences for U.S. interests when China nationalizes production and distribution and hardens its borders as it did during the COVID-19 crisis?
- What happens if Chinese government planners corner global supply alternatives?
- What happens if the United States hardens its own borders?
- What happens if U.S. allies and partners are in crisis and turn to national tools and approaches?
- What supply lines are available to the United States?
- What is current baseline U.S. production capacity and what is U.S. production capacity in the event an Administration invokes the Defense Production Act (DPA)?
- What control do chief executive officers of U.S. companies or the U.S. government have over U.S. corporate facilities and operations that are nationalized in China?
- What are U.S. dependencies on China in other critical areas such as microelectronics?
U.S. Market Competitiveness and Tariff Policy
Congress faces a series of interrelated questions about whether and how to calibrate trade policy to best position the United States in the current crisis and beyond. In response to a U.S. investigation of China's unfair trading practices under Section 301, since 2018, the United States has imposed a series of tariffs and China has responded with a series of counter tariffs that now affect a majority of trade between the two countries. Temporary tariff relief for medical supplies and pharmaceuticals could incentivize imports for the United States and other markets, but tariff policy cannot address the deeper issues of supply shortages, export constraints imposed by a number of countries including China, and product certification requirements in the United States and other markets. Tariff liberalization has been insufficient to address industrial policies within borders such as regulatory standards, procurement terms, and local content requirements that China and others impose in a range of sectors including pharmaceuticals and medical equipment.101
Recent actions by countries around the world to impose export barriers highlight potential gaps and limits to the power of WTO rules prohibiting export bans during times of global crisis. These actions also raise questions about what new rules or protocols might be needed in the future. Liberalization of U.S. import requirements also created some of the challenges the United States is facing now, such as loosening requirements for U.S. pharmaceutical firms to report on shortages and how they classify imported content for finished products that qualify as U.S. products. New liberalization could reward Chinese industrial policies in medical equipment and pharmaceuticals that seek to win new ground for Chinese firms in overseas markets. The potential for China to overwhelm global markets as it leans on exports for economic recovery raise questions about whether additional policy measures might be needed. Rather than waiting until market injury has already occurred to seek damages, for example, Congress may want to be watching trade patterns for signs of import surges and oversee the Administration's potential use of safeguard measures. Similar to the Australian government's decision on March 29, 2020 to impose new temporary restrictions on all foreign investment proposals out of concern that strategic investors—particularly those of Chinese origin—might target distressed assets, Congress may want to carefully monitor or consider whether to impose requirements about potential predatory commercial activity in the United States.
Information and Data Gaps
The outbreak of COVID-19 has exposed gaps in U.S. understanding of U.S. domestic competencies and dependencies on China and other sources of global supply. Vulnerabilities regarding raw materials, such as APIs, are not well recorded in trade and industry data. They are particularly complicated to track when materials are shipped from China and processed in a third market such as India. In similar fashion, the United States has relaxed definitions of what qualifies as a U.S. product with imported content, masking the extent to which domestically-produced products may still rely on inputs from overseas. Pharmaceutical company stockpiles are proprietary, and companies do not have to report on reserves. They are only required to report when they have a shortfall, which does not leave enough time, particularly in times of emergency, for national and contingency planning.
Under the International Investment Survey Act of 1976 (22 U.S.C. §3101 et. seq.), the President has wide authority over the collection of corporate activity abroad for statistical and analytic purposes. The Act also confers on the President the authority to request mandatory surveys of companies under specific deadlines with the ability to invoke civil and criminal penalties for noncompliance. The President has the authority to study the adequacy of current information and recommend improvements, and the Act requires him to report to Congress.102
To address these issues, Congress could consider whether to request the President to invoke his authority over the U.S. government's collection of data on corporate activity abroad. These corporate surveys could obtain specific supply chain information about the status of PPE and medical supply production, distribution, and export policy situation facing U.S. companies overseas, including in China. The surveys also could cover other sectors of potential congressional concern. This information could inform legislation that Congress has already passed or is considering with regard to overseas supply chains, including sourcing from China.
Unique Role of the U.S. Federal Government
At a time when U.S. health care systems, states, and countries overseas are seeking to secure limited medical supplies, the U.S. federal government has a unique role to play in ensuring adequate domestic and global production, contracting of supply (both domestically and globally), and distribution of these resources. Even as new capacity might be available in China, for example, who are the U.S. actors positioned to try to secure this supply and through what pathways? Lack of coordination at the federal level has led states to scramble and compete against each other for critical medical supplies in the current crisis. Among the key questions related to these issues, Congress may explore answers to such questions as:
- How does the U.S. federal government position itself vis-a-vis U.S. state and private actors?
- How does the U.S. federal government position itself vis-a-vis other foreign governments trying to secure similar supplies?
- What is the U.S. government's posture toward supplies needed in the developing world?
- How might expanded production capacity created in the United States not only help the U.S. market but also those of other countries, in the near term and over the longer term?
U.S. Leadership on Global Trade and Health Issues
The current COVID-19 pandemic provides a unique opportunity to reaffirm U.S. global leadership on trade and health issues and to counter China's nationalization and likely politicization of its domestic medical supply production capacity. China's export restraints and cornering of the global supply of medical products ahead of others in February 2020 have created serious strains on the open trade system, further incentivizing other countries to close borders and restrict any access to supplies they may have. These moves also have given China market power over other countries' procurement decisions as governments around the world grapple with how best to secure critical supplies. Early signs show that China is closely controlling and releasing supplies to other governments through contracts and some aid in ways that seek to improve China's global image and may come with other quid pro quo terms that are not yet visible. China's economic recovery ahead of others could further challenge and undermine key tenets of the open trade system, particularly if China exports pent up domestic capacity with a disregard for what the current state of the global economy is prepared to absorb on market terms.
While some European countries have imposed export restraints on their health supplies, some politicians in Europe are concerned about how the Chinese government is manipulating the crisis and China's position in global supply chains for political gain.103 Some analysts have expressed concern that China is trying to position itself as a responsible global leader in health, while violating the core tenets of WHO membership in failing to share critical information and access in the critical first few weeks as the crisis emerged in Wuhan. Members concerned about maintaining U.S. global economic leadership during the COVID-19 pandemic may consider using hearings, legislation, and statements to communicate key issues to be addressed.
Possible questions for Congress in the context of COVID-19 include:
- whether to prioritize economic openness and free flows of information;
- whether to prioritize diversifying sources of medical supplies, and if so, how;
- how best to overcome current and future bottlenecks in health care supply chains in the United States and partner nations;
- whether to respond to China's attempts to control the global narrative about key COVID-19 events, and if so, how; and
- whether to look to reform global health and trade governance in light of COVID-19 developments, and if so, how.
Some Members are calling for hearings to address the role of the WHO during the COVID-19 outbreak and are raising questions about the need to reform global health governance. Other Members are looking at the chronology of events in the COVID-19 outbreak to maintain an accurate record that is not distorted by Chinese state propaganda. Some Members are also looking at the social media platforms that the Chinese government is using to convey state propaganda—such as Twitter—and raising questions about whether this access should be allowed. Several Members have expressed an interest in potential measures to hold China accountable for its slowness to acknowledge, address, and share information regarding the outbreak of COVID-19 as H.R. 6373 required by WHO members.
Appendix A.
Bills and Resolutions Related to the Defense Production Act of 1950 (DPA)104
- 1. P.L. 116-136 - Coronavirus Aid, Relief, and Economic Security (CARES) Act
- P.L. 116-136, Section 4017 waives certain congressional oversight and reporting requirements under the Defense Production Act of 1950's (DPA; 50 U.S.C. §§4501 et seq.) Title III Expansion of Productive Capacity and Supply. Although the bulk of DPA authorities are made available at the President's discretion, Title III requires an Act of Congress for purchases or loans made to expand productive capacity in promotion of the national defense, broadly defined, for amounts greater than $50 million, and written notifications made to the relevant congressional committees of jurisdiction—the Committee on Banking, Housing, and Urban Affairs of the Senate, and the Committee on Financial Services of the House of Representatives—at least 30 days in advance. Section 4017 waives these provisions for a period of two years upon enactment. Notably, Title III already included language allowing the President to waive these requirements in a national emergency or at the non-delegable determination of the President.105
- 2. H.R. 6373 - To increase the amount available under the Defense Production Act of 1950 to respond to the coronavirus epidemic, and for other purposes.
H.R. 6373 would increase the authorized funding amount for the Defense Production Act Fund (DPA Fund) to $3 billion for FY2020-2021 from the current level of $133 million annually in response to the COVID-19 emergency. The bill also would allow for enhanced public and congressional oversight regarding the use of those funds through mandatory quarterly reporting on the use of DPA funds to congressional committees of jurisdiction, and to be made available to the public. Incorporated as a provision of H.R. 6379 - Take Responsibility for Workers and Families Act (Section 119).106
- 1. H.R. 6399 - To amend the Defense Production Act of 1950 to ensure the supply of certain medical articles essential to national defense, and for other purposes.
H.R. 6399 would amend the DPA statute to fortify industry production of medical resources in response to the COVID-19 emergency.107
- 1. S. 3568 - A bill to require the President to use authorities under the Defense Production Act of 1950 to require emergency production of medical equipment to address the COVID-19 outbreak.
S. 3568 would seek to compel the President to exercise the Defense Production Act for the development of specific medical equipment, including: N95 respirators, medical ventilators, face shields, medical exam gloves, surgical gowns, and other medical equipment as needed to respond to the COVID-19 emergency. The bill would also compel the President to establish a price on those goods. This bill is the Senate companion bill to H.R. 6390.108
- 1. S.Res. 547 - A resolution encouraging the President to use authorities provided by the Defense Production Act of 1950 to scale up the national response to the coronavirus crisis.
S.Res. 547 calls upon the President to exercise Defense Production Act authorities to increase production of medical supplies, including personal protective equipment, to respond to the COVID-19 emergency. It supports the use of such authorities to: (1) distribute medical materials, including by directing suppliers to prioritize and accept contracts to restock the Strategic National Stockpile; and (2) establish voluntary agreements and provide financial incentives to manufacturers and suppliers of critical medical equipment.109
- 1. S. 3570 - A bill to provide for the expedited procurement of equipment needed to combat COVID-19 under the Defense Production Act of 1950.
- 2. S. 3570 would trigger the breadth of authorities under the DPA to effect: a major purchase order for 300 million N95 masks; requires the National Response Coordination Center to conduct a national assessment on current medical supply needs and a follow up major purchase order to fulfill the needs identified in the assessment; waive restrictions on dollar limitations for orders executed under DPA and a 30 day waiting period for orders that exceed $50 million; and authorize increased funding for DPA accounts that are being considered for supplemental COVID-19 spending packages.110
- 3. H.Res. 906 - Calling on the President to invoke the Defense Production Act to respond to COVID-19.
H.Res. 906 calls on the President to: (1) use all relevant authorities of the Defense Production Act to direct the domestic production of supplies to address COVID-19; and (2) share specified information regarding the use of such authorities with Congress. The resolution also states that Congress stands ready to make additional appropriations available for this effort.111
- 1. H.R. 6398 - To provide for the expedited procurement of equipment needed to combat COVID-19 under the Defense Production Act of 1950.
H.R. 6398 is the companion bill to S. 3570, which would trigger the breadth of authorities under the DPA to effect: a major purchase order for 300 million N95 masks; requires the National Response Coordination Center to conduct a national assessment on current medical supply needs and a follow up major purchase order to fulfill the needs identified in the assessment; waive restrictions on dollar limitations for orders executed under DPA and a 30 day waiting period for orders that exceed $50 million; and authorize increased funding for DPA accounts that are being considered for supplemental COVID-19 spending packages.112
- 1. H.R. 6390 - To require the President to use authorities under the Defense Production Act of 1950 to require emergency production of medical equipment to address the COVID-19 outbreak.
H.R. 6390 would seek to compel the President to exercise the Defense Production Act for the development of specific medical equipment, including: N95 respirators, medical ventilators, face shields, medical exam gloves, surgical gowns, and other medical equipment as needed to respond to the COVID-19 emergency. The bill would also compel the President to establish a price on those goods. This bill is the House companion bill to S. 3568.113
Appendix B.
U.S. Imports of Select Medical Products
HS Code 9019.20
| CT Scanners HS Code 9022.12
| ||||||
Medical Protective Articles of Textile Materials (Including Masks) HS Code 6307.90
| Digital and Infrared Thermometers HS Code 9025.19
|
Source: CRS using data from the U.S. International Trade Commission's DataWeb and Global Trade Atlas.
|
Economy |
Value (US$) |
|
Ireland |
35,797,919,666 |
|
Germany |
25,416,992,979 |
|
China |
20,744,036,029 |
|
Switzerland |
19,115,982,191 |
|
Mexico |
15,758,366,376 |
|
Italy |
9,356,424,042 |
|
Canada |
9,072,982,790 |
|
India |
8,325,151,620 |
|
Japan |
8,126,636,035 |
|
Singapore |
7,947,308,765 |
|
Rest of the World |
64,911,471,228 |
|
Total |
$224,573,271,721 |
Source: CRS using the World Customs Organization's "HS Classification Reference for COVID-19 Medical Supplies;" Gary Clyde and Jeffrey J. Schott's "List of Pharmaceutical and Medical Device Products by Harmonized System (HS) Code" in Local Content Requirements: A Global Problem; and Chad Bown's "Trump's Trade Policy Is Hampering the U.S. Fight Against COVID-19." Data sourced from the U.S. International Trade Commission's DataWeb and Global Trade Atlas.
Notes: The figures presented here cover product categories at the HTS six-digit level.
Author Contact Information
Footnotes
| 1. |
Finbarr Bermingham and Su-Lin Tan, "Coronavirus: China's mas-making juggernaut cranks into gear, sparking fears of over-reliance on world's workshop," South China Morning Post, March 12, 2020, https://www.scmp.com/economy/global-economy/article/3074821/coronavirus-chinas-mask-making-juggernaut-cranks-gear; U.S. Food and Drug Administration, "Coronavirus (COVID-19) Supply Chain Update," Press release, February 27, 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update. |
| 2. |
Zhang Pinghui and Zhou Xin, "Coronavirus: China Shifts Responsibility Over Medical Supplies Amid Mask Shortage, Rising Death Toll," South China Morning Post, February 3, 2020, updated on February 14, 2020, https://www.scmp.com/economy/china-economy/article/3048744/coronavirus-mask-shortage-prompts-beijing-tweak-authority; Finbarr Bermingham and Su-Lin Tan, "Coronavirus: China's Mask Making Juggernaut Cranks Into Gear, Sparking Fears of Overreliance on World's Workshop," South China Morning Post, March 12, 2020, https://www.scmp.com/economy/global-economy/article/3074821/coronavirus-chinas-mask-making-juggernaut-cranks-gear; Engen Tham, Cheng Leng, and Zhang Yan, "Exclusive: Unilever, 3M on List of Firms Eligible for China Loans to Ease Coronavirus Crisis—Sources," Reuters, February 19, 2020, https://www.reuters.com/article/us-china-health-lending-exclusive-idUSKBN20D0SQ; Yang Jian, "GM, Wuling Venture Begins Output of Machines to Make Face Masks, Automotive News, February 20, 2020, https://www.autonews.com/china/gm-wuling-venture-begins-output-machines-make-face-masks; and Luffy Liu, "700 Tech Companies in China Have Begun Making Masks," EE Times, February 13, 2020, https://www.eetimes.com/700-tech-companies-in-china-have-begun-making-masks/. |
| 3. |
"Circular on Further Facilitating the Import and Export of Technology During the Period of Epidemic Prevention and Control," PRC Ministry of Commerce, February 4, 2020, http://english.mofcom.gov.cn/article/newsrelease/significantnews/202002/20200202934774.shtml; and "Circular on Actively Expanding Imports to Combat Against Novel Coronavirus Epidemic," PRC Ministry of Commerce, February 6, 2020, http://english.mofcom.gov.cn/article/newsrelease/significantnews/202002/20200202. |
| 4. |
Li Yan, "Xi Says China to Send More Medical Experts to Italy," Xinhua, March 17, 2020, http://www.ecns.cn/m/news/politics/2020-03-17/detail-ifzunmih1236562.shtml934157.shtml, and "'Mask Diplomacy' From Beijing to Change Narrative About COVID-19," SupChina, March 23, 2020, https://supchina.com/2020/03/23/mask-diplomacy-from-beijing-to-change-narrative-about-covid-19/. |
| 5. |
Norihiko Sirouzu and Yilei Sun, "As China's 'Detroit' Reopens, World's Automakers Worry About Disruptions," Reuters, March 8, 2020. |
| 6. |
CRS calculations based on data from the U.S. Department of Commerce, Bureau of Economic Analysis, "Gross Domestic Product, Fourth Quarter and Year 2019 (Second Estimate)," and "U.S. International Trade in Goods and Services, January 2020." Total U.S.-China trade amounted to $635.3 billion in 2019. |
| 7. |
The following data is sourced from the U.S. Department of Commerce, Bureau of Economic Analysis' International Transactions. |
| 8. |
Ryan Woo, Se Young Lee, David Stanway, and Andrew Galbraith, "Goldman Sees China's Economy Shrinking 9 Percent in First Quarter Amid Coronavirus Outbreak," Reuters, March 16, 2020, https://www.reuters.com/article/us-health-coronavirus-china-toll/goldman-sees-chinas-economy-shrinking-9-in-first-quarter-amid-coronavirus-outbreak-idUSKBN21340T. |
| 9. |
"Apple Supplier Foxconn Expects Coronavirus-Hit Labor Shortage in China to Ease," The Wall Street Journal, March 3, 2020. |
| 10. |
Duncan Riley, "Apple and Others May Have Avoided Supply Shortages as some Foxconn Plants Reopen in China," Silicon Angle, February 11, 2020. |
| 11. |
Alistair MacDonald, "Steelmakers Rely Heavily on China," The Wall Street Journal, March 6, 2020. |
| 12. |
Samantha McDonald, "Columbia, Burberry and 12 Other Fashion Firms Are Closing Stores Due to Coronavirus," Footwear News, February 27, 2020. |
| 13. |
Hayley Peterson, "Starbucks Reopens Most Stores in China, Citing 'Early Signs of Recovery,' From Coronavirus," Business Insider, February 27, 2020. |
| 14. |
United Nations World Tourism Organization, "Exports from International Tourism Hit USD 1.7 Trillion," June 6, 2019, https://www.unwto.org/global/press-release/2019-06-06/exports-international-tourism-hit-usd-17-trillion; U.S. International Trade Administration National Travel and Tourism Office, "Fast Facts: United States Travel and Tourism Industry 2018," October 2019, https://travel.trade.gov/outreachpages/download_data_table/Fast_Facts_2018.pdf. |
| 15. |
Dawn Gilbertson, "Coronavirus Travel Fallout: American, Delta Cutting Flights as Demand Sinks, Joining United and Other," USA Today, March 10, 2020. |
| 16. |
"FedEx, UPS Warn of Delivery Delays, JPMorgan Tests Virus Contingency Plan," PYMNTS.com, March 3, 2020. |
| 17. |
Ministry of Foreign Affairs of the People's Republic of China, "CAAC to Further Reduce International Passenger Flights," press release, March 27, 2020, https://www.fmprc.gov.cn/mfa_eng/topics_665678/kjgzbdfyyq/t1762623.shtml. |
| 18. |
Margot Roosevelt, "Truckers, Dockworkers Suffer as Coronavirus Chokes L.A., Long Beach Ports Cargo, Los Angeles Times, March 7, 2020. |
| 19. |
Lee Hong Liang, "Port Authority of New York and New Jersey seeks $1.9bn bailout amid COVID-19," Seatrade Maritime News, March 25, 2020; Port Authority of New York and New Jersey, Letter to Members of the New York and New Jersey Congressional Delegations, letter, March 19, 2020, https://www.politico.com/states/f/?id=00000170-fadd-d9d1-a3f3-fbdd91ed0000. |
| 20. |
Jacob Bunge, "Meat Stockpiles Surge as Coronavirus Epidemic Curbs Exports," The Wall Street Journal, March 2, 2020. |
| 21. |
Trinh Nguyen, "The Economic Fallout of the Coronavirus in Southeast Asia," Carnegie Endowment for International Peace, February 13, 2020. |
| 22. |
"China virus outbreak threatens global drug supplies: European business group," Reuters, February 17, 2020. |
| 23. |
Office of the United States Trade Representative, "Economic and Trade Agreement Between the Government of the United States of America and the Government of the People's Republic of China," January 15, 2020. |
| 24. |
National Development and Reform Commission of the People's Republic of China, "关于2020年粮食棉花进口关税配额申请和分配细则的公告 2019年第9号 (Announcement Regarding Application and Distribution Rules for Import Tariff Rate Quotas for Grain and Cotton)" September 29, 2019, https://www.ndrc.gov.cn/xxgk/zcfb/gg/201909/t20190930_1181887.html. |
| 25. |
CRS In Focus IF11342, China's Corporate Social Credit System, by Michael D. Sutherland. |
| 26. |
Austin Ramzy, "U.S. Lawmakers Propose Tough Limits on Imports from Xinjiang," The New York Times, March 11, 2020. |
| 27. |
Office of the United States Trade Representative, "Economic and Trade Agreement Between the Government of the United states of America and the Government of the People's Republic of China," January 15, 2020, Article 6.2. |
| 28. |
Zhou Xin, "Coronavirus: Doubts Raised Over Whether Chinese Companies Can Use Force Majeure to Counter Risks," South China Morning Post, February 25, 2020, https://www.scmp.com/economy/china-economy/article/3052277/coronavirus-doubts-raised-over-whether-chinese-companies-can. |
| 29. |
Dan Harris, "Force Majeure in the Time of Coronavirus," China Law Blog, Harris Bricken, February 27, 2020, https://www.chinalawblog.com/2020/02/force-majeure-in-the-time-of-coronavirus.html. |
| 30. |
Finbarr Bermingham and Su-Lin Tan, "Coronavirus: China's mas-making juggernaut cranks into gear, sparking fears of over-reliance on world's workshop," South China Morning Post, March 12, 2020, https://www.scmp.com/economy/global-economy/article/3074821/coronavirus-chinas-mask-making-juggernaut-cranks-gear; U.S. Food and Drug Administration, "Coronavirus (COVID-19) Supply Chain Update," Press release, February 27, 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update. |
| 31. |
Zhang Pinghui and Zhou Xin, "Coronavirus: China Shifts Responsibility Over Medical Supplies Amid Mask Shortage, Rising Death Toll," South China Morning Post, February 3, 2020, updated on February 14, 2020, |
| 32. |
"Exclusive: Unilever, 3M, on list of firms eligible for China loans to ease coronavirus crisis – sources," Reuters, February 13, 2020, https://www.reuters.com/article/us-china-health-lending-exclusive-idUSKBN20D0SQ; Yang Jian, "GM, Wuling venture begins output of machines to make face masks," Automotive News China, February 20, 2020, https://www.autonews.com/china/gm-wuling-venture-begins-output-machines-make-face-masks. |
| 33. |
Zhang Pinghui and Zhou Xin, "Coronavirus: China Shifts Responsibility Over Medical Supplies Amid Mask Shortage, Rising Death Toll," South China Morning Post, February 3, 2020, updated on February 14, 2020, https://www.scmp.com/economy/china-economy/article/3048744/coronavirus-mask-shortage-prompts-beijing-tweak-authority; Finbarr Bermingham and Su-Lin Tan, "Coronavirus: China's Mask Making Juggernaut Cranks Into Gear, Sparking Fears of Overreliance on World's Workshop," South China Morning Post, March 12, 2020, https://www.scmp.com/economy/global-economy/article/3074821/coronavirus-chinas-mask-making-juggernaut-cranks-gear; Engen Tham, Cheng Leng, and Zhang Yan, "Exclusive: Unilever, 3M on List of Firms Eligible for China Loans to Ease Coronavirus Crisis—Sources," Reuters, February 19, 2020, https://www.reuters.com/article/us-china-health-lending-exclusive-idUSKBN20D0SQ; Yang Jian, "GM, Wuling Venture Begins Output of Machines to Make Face Masks, Automotive News, February 20, 2020, https://www.autonews.com/china/gm-wuling-venture-begins-output-machines-make-face-masks; and Luffy Liu, "700 Tech Companies in China Have Begun Making Masks," EE Times, February 13, 2020, https://www.eetimes.com/700-tech-companies-in-china-have-begun-making-masks/. |
| 34. |
Ministry of Commerce of the People's Republic of China, "Circular on Further Facilitating the Import and Export of Technology During the Period of Epidemic Prevention and Control," February 4, 2020, http://english.mofcom.gov.cn/article/newsrelease/significantnews/202002/20200202934774.shtml; and Ministry of Commerce of the People's Republic of China, "Circular on Actively Expanding Imports to Combat Against Novel Coronavirus Epidemic," February 6, 2020, http://english.mofcom.gov.cn/article/newsrelease/significantnews/202002/20200202934157.shtml. |
| 35. |
Ministry of Commerce of the People's Republic of China, "General Office of the Ministry of Commerce Issued the Circular on Actively Expanding Imports to Combat against Novel Coronavirus Epidemic," press release, February 6, 2020. |
| 36. |
Phillip Coorey, "China Spree Sparks FIRB Crackdown," Financial Review, March 29, 2020, https://www.afr.com/politics/federal/china-spree-sparks-firb-crackdown-20200329-p54exo. |
| 37. |
Kate McKlymont, "Second Developer Flew 82 Tonnes of Medical Supplies to China," The Sydney Morning Herald, March 26, 2020, https://www.smh.com.au/national/second-developer-flies-82-tonnes-of-medical-supplies-to-china-20200326-p54e8n.html. |
| 38. |
Kate McKlymont, "Second Developer Flew 82 Tonnes of Medical Supplies to China," The Sydney Morning Herald, March 26, 2020, https://www.smh.com.au/national/second-developer-flies-82-tonnes-of-medical-supplies-to-china-20200326-p54e8n.html. |
| 39. |
Keith Bradsher and Liz Alderman, "The World Needs Masks. China Makes Them—But Has Been Hoarding Them," The New York Times, March 16, 2020, https://www.nytimes.com/2020/03/13/business/masks-china-coronavirus.html. |
| 40. |
"China Imposes No Export Ban on Masks: Commerce Official," Xinhua, March 5, 2020, http://english.www.gov.cn/statecouncil/ministries/202003/05/content_WS5e60c25dc6d0c201c2cbda0b.html. |
| 41. |
Executive Order 13909 "Prioritizing and Allocating Health and Medical Resources to Respond to the Spread of COVID-19," 85 Federal Register 56, March 23, 2020, https://www.govinfo.gov/content/pkg/FR-2020-03-23/pdf/2020-06161.pdf; CRS Report R43767, The Defense Production Act of 1950: History, Authorities, and Considerations for Congress, by Michael H. Cecire and Heidi M. Peters; CRS Insight IN11231, The Defense Production Act (DPA) and COVID-19: Key Authorities and Policy Considerations, by Michael H. Cecire and Heidi M. Peters; CRS Insight IN11280, COVID-19: Industrial Mobilization and Defense Production Act (DPA) Implementation, by Michael H. Cecire and Heidi M. Peters. |
| 42. |
CRS Insight IN11231, The Defense Production Act (DPA) and COVID-19: Key Authorities and Policy Considerations, by Michael H. Cecire and Heidi M. Peters |
| 43. |
The White House, "Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing," March 22, 2020, https://www.whitehouse.gov/briefings-statements/remarks-president-trump-vice-president-pence-members-coronavirus-task-force-press-briefing-8/. |
| 44. |
Lena H. Sun and Rachel Siegel, "As Demand Spikes for Medical Equipment, This Texas Manufacturer is Caught in the Coronavirus Supply Chain Panic, The Washington Post, February 15, 2020, https://www.washingtonpost.com/business/2020/02/15/coronavirus-mask-shortage-texas-manufacturing/. |
| 45. |
Aaron Gregg, Dan Lamothe, and Christian Davenport, "Having Automakers Churn Out Ventilators Won't be Quick or Easy, Experts Say," The Washington Post, March 21, 2020. |
| 46. |
U.S. Embassy & Consulates in China, "The United States Announces Assistance to the COVID-19," U.S. Department of State, press release, February 7, 2020, https://china.usembassy-china.org.cn/the-united-states-announces-assistance-to-the-novel-coronavirus/; UPS Foundation, "UPS to Airlift More Than 2 Million Masks and Protective Gear To China," UPS Pressroom, press release, January 31, 2020, https://pressroom.ups.com/pressroom/ContentDetailsViewer.page?ConceptType=PressReleases&id=1580479415168-269&WT.mc_id=UPSCOM_NEWSANDINFO_CORONA_PRESSRELEASE_013120; Direct Relief, "Direct Relief Rushes Facial Masks to China to Fight Coronavirus Spread," press release, January 28, 2020, https://www.directrelief.org/2020/01/direct-relief-rushes-facial-masks-to-china-to-fight-coronavirus-spread/; Tad Walch, "Inside the church's donation of masks, coveralls and goggles to China over the coronavirus outbreak," Deseret News, January 29, 2020, https://www.deseret.com/faith/2020/1/29/21113386/coronavirus-china-outbreak-lds-church-mormon-russell-nelson-donation-chinese-health-wuhan. |
| 47. |
William Mauldin, "U.S. to Allow Some Delays in Tariff Payments," The Washington Post, March 26, 2020. |
| 48. |
"Notice of Product Exclusions: China's Acts, Policies and Practices Related to Technology Transfer, Intellectual Property, and Innovation," USTR Notice, U.S. Federal Register, March 10, 2020, https://www.federalregister.gov/documents/2020/03/10/2020-05000/notice-of-product-exclusions-chinas-acts-policies-and-practices-related-to-technology-transfer. Also see https://www.govinfo.gov/content/pkg/FR-2020-03-10/pdf/2020-05000.pdf and |
| 49. |
Robert E. Lighthizer, "Lighthizer Responds: Medical Trade Tariffs," Letter to the Editor, The Wall Street Journal, March 21-22, 2020. |
| 50. |
Alex Leary and William Mauldin, "Import Tariffs Will be Halted, Officials Say," The Wall Street Journal, March 28-29, 2020. |
| 51. |
"Request for Comments Concerning the Extension of Particular Extensions Granted Under the June 2019 Product Exclusion Notice From the $34 Billion Action Pursuant to Section 301: China's Acts, Policies and Practices Related to Technology Transfer, Intellectual Property and Innovation," USTR Notice, U.S. Federal Register, March 20, 2020, https://www.federalregister.gov/documents/2020/03/20/2020-05890/request-for-comments-concerning-the-extension-of-particular-exclusions-granted-under-the-june-2019. |
| 52. |
Jonathan Swan and Joann Muller, "Inside the Start of the Great Virus Airlift," Axios, March 29, 2020, https://www.axios.com/coronavirus-airlift-masks-medical-supplies-1d1913bf-744e-41cf-895c-d8934afa2c36.html?utm_source=newsletter&utm_medium=email&utm_campaign=newsletter_axiossneakpeek&stream=top. |
| 53. |
Testimony of FDA Associate Commissioner for Global Policy and Strategy Mark Abdoo, in U.S. China Security and Economic Review Commission, Exploring the Growing Reliance on China's Biotech and Pharmaceutical Products, July 31, 2019, https://www.fda.gov/news-events/congressional-testimony/exploring-growing-us-reliance-chinas-biotech-and-pharmaceutical-products-07312019. Information provided by FDA's Office of Legislation through personal communication with CRS. FDA's usage of the term "import line" refers to a distinct regulated product within a shipment through customs. |
| 54. |
FDA, Testimony of Dr. Janet Woodcock, Director the Center for Drug Evaluation and Research, "Securing the U.S. Drug Supply Chain: Oversight of FDA's Foreign Inspection Program," December 10, 2019. |
| 55. |
Stephanie Findlay, Hannah Kuchler, and Sarah Neville, "Drugmakers braced for coronavirus disruption to China supplies," Financial Times, February 12, 2020, https://www.ft.com/content/8630c51c-4cc0-11ea-95a0-43d18ec715f5; Reuters, "China Virus Outbreak Threatens Global Drug Supplies: European business group," February 17, 2020, https://www.reuters.com/article/us-china-health-pharma-antibiotics/china-virus-threatens-global-antibiotics-supply-european-business-group-idUSKBN20C08S. |
| 56. |
CRS calculations using data from the U.S. International Trade Commission's DataWeb (HTSUS 2941.30 and 2941.10). |
| 57. |
U.S. Food and Drug Administration, "Coronavirus (COVID-19) Supply Chain Update," Press release, February 27, 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update. |
| 58. |
Food and Drug Administration, "Frequently Asked Questions about Drug Shortages," last updated July 05, 2018, https://www.fda.gov/drugs/drug-shortages/frequently-asked-questions-about-drug-shortages#q7; and CDER Office of Compliance Office of Drug Security, Integrity & Recalls, "FDA Compliance Focal Point for Imports & Exports of CDER Regulated Drugs, https://www.fda.gov/media/91681/download. |
| 59. |
Yanzhong Huang, "The Coronavirus Outbreak Could Disrupt the U.S. Drug Supply," Council on Foreign Relations, March 5, 2020, https://www.cfr.org/in-brief/coronavirus-disrupt-us-drug-supply-shortages-fda; Julianna Tatelbaum, "Fears of US drug shortages grow as India locks down to curb the coronavirus," CNBC, March 24, 2020. |
| 60. |
Reuters, "Global supplier India curbs drug exports as coronavirus fears grow," March 3, 2020. |
| 61. |
Bryce Baschuk, "Export Bans on Medical Supplies to Hamper Global Virus Response," Bloomberg, March 18, 2020. Some public health officials and trade experts have also expressed concern that export controls and other restrictions could reduce incentives for companies to ramp up production. See, for example, James Politi, Aime Williams, and Clive Cookson, "US official hits out at hoarding of coronavirus medical supplies," Financial Times," March 5, 2020. |
| 62. |
World Trade Organization, "COVID-19 and World Trade," last updated March 31, 2020, https://www.wto.org/english/tratop_e/covid19_e/covid19_e.htm. |
| 63. |
G20 Leaders' Statement, "Extraordinary G20 Leaders Summit Statement on COVID-19," March 30, 2020, https://g20.org/en/media/Documents/G20_Extraordinary%20G20%20Leaders%E2%80%99%20Summit_Statement_EN%20(3).pdf. |
| 64. |
Lili Bayer, Jillian Deutsch, Jakob Hanke Vale, and Paola Tamma, "EU Moves To Limit Exports of Medical Equipment Outside the Bloc," Politico, March 15, 2020. |
| 65. |
Simon J. Evenett, Tackling Coronavirus: The Trade Policy Dimension, Swiss Institute of International Economics and Department of Economics, University of St. Gallen, Switzerland, March 10, 2020. Some countries, including Australia, Brunei, Canada, Chile, Myanmar, New Zealand and Singapore, have pledged "to keep trade lines open." Andrea Shalal, "U.S. Should Refrain from Export Controls in Pandemic Response: Chamber of Commerce," Reuters, March 25, 2020. |
| 66. |
CRS calculations using data from the U.S. International Trade Commission's DataWeb and Global Trade Atlas. |
| 67. |
Chen Aizhu and Tom Daly, "Where's the Data? Angst for Commods Traders as China Trade Figures Held in Limbo," Reuters, June 1, 2018, https://www.reuters.com/article/us-usa-trade-china-data/wheres-the-data-angst-for-commods-traders-as-china-trade-figures-held-in-limbo-idUSKCN1IX4LQ. |
| 68. |
Ryan Woo, Se Young Lee, David Stanway, and Andrew Galbraith, "Goldman Sees China's Economy Shrinking 9 Percent in First Quarter Amid Coronavirus Outbreak," Reuters, March 16, 2020, https://www.reuters.com/article/us-health-coronavirus-china-toll/goldman-sees-chinas-economy-shrinking-9-in-first-quarter-amid-coronavirus-outbreak-idUSKBN21340T. |
| 69. |
Hua Xia, "China Advances Streamlining Approval Procedures, Fostering New Growth Drivers to Keep Employment Stable," Xinhua, March 17, 2020, http://www.xinhuanet.com/english/2020-03/17/c_138888715.htm. |
| 70. |
Ryan Woo, Se Young Lee, David Stanway, and Andrew Galbraith, "Goldman Sees China's Economy Shrinking 9 Percent in First Quarter Amid Coronavirus Outbreak," Reuters, March 16, 2020, https://www.reuters.com/article/us-health-coronavirus-china-toll/goldman-sees-chinas-economy-shrinking-9-in-first-quarter-amid-coronavirus-outbreak-idUSKBN21340T. |
| 71. |
John Burn-Murdoch, Cale Tilford, Steven Bernard, Keith Fray, and Alan Smith, "Coronavirus Economic Tracker: Latest Global Fallout," Financial Times, accessed March 26, 2020; and "Coronavirus: Getting China Back to Work," Trivium China, last updated April 1, 2020, https://triviumchina.com/2020/03/07/coronavirus-getting-china-back-to-work/. |
| 72. |
Li Yan, "Wuhan Companies Begin to Resume Production," China Daily, March 17, 2020, http://www.ecns.cn/m/business/2020-03-17/detail-ifzunmih1236408.shtml. |
| 73. |
"MOFCOM VM Wang Shouwen Holds Back to Work Call," American Chamber of Commerce in China, March 19, 2020, https://www.amchamchina.org/news/mofcom-vm-wang-shouwen-holds-back-to-work-call; and "European Chamber's Conference Call with the Vice Minister of Commerce Wang Shouwen Joined by Multiple Departments on COVID-19's Impact to FIEs," European Chamber of Commerce in China, March 13, 2020, https://www.europeanchamber.com.cn/en/lobby-actions/3949/European_Chamber_s_Conference_Call_with_Vice_Minister_of_Commerce_Wang_Shouwen_Joined_by_Multiple_Departments_on_COVID_19_Impacts_to_FIEs. |
| 74. |
Yukon Huang, "China's Economic Growth Now Depends on the West," Carnegie Endowment for International Peace, March 19, 2020, https://carnegieendowment.org/2020/03/19/china-s-economic-growth-now-depends-on-west-pub-81326. |
| 75. |
"China January-February Exports Tumble, Imports Down as Coronavirus Batters Trade and Business," Reuters, March 6, 2020, https://www.reuters.com/article/us-china-economy-trade/china-january-february-exports-tumble-imports-down-as-coronavirus-batters-trade-and-business-idUSKBN20U05R. |
| 76. |
Anindya Barman Zacks," China Steel Output Rises Despite Oversupply and Coronavirus," Yahoo Finance, March 19, 2020. |
| 77. |
"World Steel in Figures 2019," World Steel Association, https://www.worldsteel.org/media-centre/press-releases/2019/world-steel-in-figures-2019.html; Min Zhang and Tom Daly, "China 2019 Crude Steel Output Jumps 8.3%, Sets Second Straight Annual Record," Reuters, January 16, 2020. |
| 78. |
"Announcement on Increasing the Export Tax Rebate Rate for Some Products," PRC Ministry of Finance Announcement No. 15, March 17, 2020, http://www.chinatax.gov.cn/chinatax/n810341/n810755/c5146338/content.html. |
| 79. |
"China's Legislature Adopts Decision on Banning Illegal Trade, Consumption of Wildlife," Xinhua, February 24, 2020, http://www.xinhuanet.com/english/2020-02/24/c_138814328.htm. |
| 80. |
Robert D. Atkinson, "China's Biopharmaceutical Strategy: Challenge or Complement to U.S. Industry Competitiveness?" ITIF, August 12, 2019, https://itif.org/publications/2019/08/12/chinas-biopharmaceutical-strategy-challenge-or-complement-us-industry. |
| 81. |
Gao Yu, Peng Yanfeng, Yang Rui, Feng Yuding, Ma Danmeng, Flynn Murphy, Han Wei, and Timmy Shen, "In Depth: How Early Signs of a SARS-Like Virus Were Spotted, Spread, and Throttled," Caixin, February 29, 2020, https://www.caixinglobal.com/2020-02-29/in-depth-how-early-signs-of-a-sars-like-virus-were-spotted-spread-and-throttled-101521745.html; and Huang Shulun, Huang Huizhao, Peng Yanfeng, Liu Yuan, and Tang Ziyi, "Destroyed Market Samples Make it Impossible to Trace Origin of Deadly Virus, Experts Say," Caixin, February 8, 2020, https://www.caixinglobal.com/2020-02-08/destroyed-market-samples-make-it-impossible-to-trace-origin-of-deadly-virus-expert-says-101513162.html. |
| 82. |
"China Inaugurates the First Biocontainment Level 4 Laboratory in Wuhan," Wuhan Institute of Virology, China Academy of Sciences, February 3, 2015, http://english.whiov.cas.cn/News/Events/201502/t20150203_135923.html. |
| 83. |
"Report of the WHO-China Joint Mission on Coronavirus 2019 (COVID-19), February 16-24, 2020, https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. |
| 84. |
Jay Barmann, "Bay Area-Based Gilead Sees Potential Legal Conflict with China Over its Coronavirus Drug," SFIST, February 6, 2020, https://sfist.com/2020/02/06/bay-area-based-gilead-donates-experimental-anti-viral-drug-to-china/; and Elise Mak, "Gilead's Remdesivir Enters China Phase III Trial to Fight Coronavirus," BioWorld, February 3, 2020, https://www.bioworld.com/articles/432804-gileads-remdesivir-enters-china-phase-iii-trial-to-fight-coronavirus. |
| 85. |
"Chinese Biotech Censured for False Claim on Gilead's Virus Drug," Bloomberg News, March 1, 2020, https://www.bloomberg.com/news/articles/2020-03-02/chinese-biotech-censured-for-false-claim-on-gilead-s-virus-drug; and Joe McDonald and Linda A. Johnson, "Chinese Scientists Ask for Patent on U.S. Drug to Fight Virus," Associated Press, February 6, 2020, https://apnews.com/1fe943717b56b56cce5e733790f016dd. |
| 86. |
Reuters, "Jointown Pharmaceutical To Issue Up To 1.5 Bln Yuan Worth of Renewable Bonds," March 4, 2020. |
| 87. |
"Vision and Actions on Jointly Building the Silk Road Economic Belt and 21stCentury Maritime Silk Road," National Development and Reform Commission, Ministry of Foreign Affairs, and the Ministry of Commerce, March 28, 2015, https://reconasia-production.s3.amazonaws.com/media/filer_public/e0/22/e0228017-7463-46fc-9094-0465a6f1ca23/vision_and_actions_on_jointly_building_silk_road_economic_belt_and_21st-century_maritime_silk_road.pdf. |
| 88. |
Li Yan, "Xi Says China to Send More Medical Experts to Italy," Xinhua, March 17, 2020, http://www.ecns.cn/m/news/politics/2020-03-17/detail-ifzunmih1236562.shtml. |
| 89. |
James Kraska, "China is Legally Responsible for COVID-19 Damage and Claims Could be in the Trillions," War on the Rocks, March 23, 2020, https://warontherocks.com/2020/03/china-is-legally-responsible-for-covid-19-damage-and-claims-could-be-in-the-trillions/. |
| 90. |
Cheng Ting-Fang and Lauly Li, "China Lets Wuhan Tech Plants Bypass Lockdown to Stay Open," Nikkei Asian Review, March 4, 2020, https://asia.nikkei.com/Spotlight/Coronavirus/China-lets-Wuhan-tech-plants-bypass-lockdown-to-stay-open. |
| 91. |
"Huawei, Chinese Chip Makers Keep Factories Humming, Despite the Virus," Reuters, February 2, 2020, https://www.reuters.com/article/us-china-health-tech-huawei/huawei-chinese-chip-makers-keep-factories-humming-despite-virus-outbreak-idUSKBN1ZX0CX. |
| 92. |
Dan Strumpf, "Huawei Staff Return After Outbreak," The Wall Street Journal, March 26, 2020. |
| 93. |
Li Yan, "Xi Says China to Send More Medical Experts to Italy," Xinhua, March 17, 2020, http://www.ecns.cn/m/news/politics/2020-03-17/detail-ifzunmih1236562.shtml934157.shtml; Reuters, China sends Serbia help to halt coronavirus spreading," March 21, 2020. |
| 94. |
Wendy Wu, "Coronavirus: Don't Politicize Medical Supply Problems, China Says," South China Morning Post, March 30, 2020, https://www.scmp.com/news/china/diplomacy/article/3077537/coronavirus-chinas-ambassador-closely-following-netherlands. |
| 95. |
"Barriers Should be Removed to Send Medical Supplies to US," Global Times, March 24, 2020, https://www.globaltimes.cn/content/1183647.shtml. |
| 96. |
Keith Bradsher and Liz Alderman, "The World Needs Masks. China Makes Them—But Has Been Hoarding Them," The New York Times, March 16, 2020, https://www.nytimes.com/2020/03/13/business/masks-china-coronavirus.html. |
| 97. |
Individual bill text not available as of April 3, 2020. Details available at Office of Senator Marco Rubio, "Rubio, Colleagues Introduce the Strengthening America's Supply Chain and National Security Act," press release, March 19, 2020, https://www.rubio.senate.gov/public/index.cfm/2020/3/rubio-colleagues-introduce-the-strengthening-america-s-supply-chain-and-national-security-act. |
| 98. |
Individual bill text not available as of April 3, 2020. Details available at Office of Senator Tom Cotton, "Moving American Pharmaceutical Production Out of China for the Health and Safety of Americans," March 18, 2020, https://www.cotton.senate.gov/files/documents/Cotton%20Gallagher%20Bill%20FINAL.pdf. |
| 99. |
Published bill text not available as of April 3, 2020. Draft text available at Office of Congressman Matt Gaetz, "Congressman Matt Gaetz Introduces "No CHINA Act" to Prevent Funneling of COVID-19 Relief Funds to Businesses Owned by Chinese Government," press release, March 24, 2020, https://gaetz.house.gov/media/press-releases/congressman-matt-gaetz-introduces-no-china-act-prevent-funneling-covid-19. |
| 100. |
"White House Working on Order to Cut U.S. Dependency on Foreign Medicines," Reuters, March 16, 2020. |
| 101. |
Jennifer A. Hillman, "Six Proactive Steps in a Smart Trade Approach to Fighting COVID-19," ThinkGlobalHealth, March 20, 2020, https://www.thinkglobalhealth.org/article/six-proactive-steps-smart-trade-approach-fighting-covid-19 Anabel Gonzalez, "A Memo to Trade Ministers on How Trade Policy Can Fight COVID-19," PIEE, March 23, 2020, https://www.piie.com/blogs/trade-and-investment-policy-watch/memo-trade-ministers-how-trade-policy-can-help-fight-covid. |
| 102. |
https://uscode.house.gov/view.xhtml?path=/prelim@title22/chapter46&edition=prelim; and "Legal Authority and Confidentiality of International Survey Collections," U.S. Bureau of Economic Analysis, https://www.bea.gov/about/legal-authority-and-confidentiality-international-survey-collections. |
| 103. |
"The Coronavirus and the New World it is Creating," European Union External Action Office, March 23, 2020, https://eeas.europa.eu/headquarters/headquarters-homepage_en/76379/The%20Coronavirus%20pandemic%20and%20the%20new%20world%20it%20is%20creating. |
| 104. |
50 U.S.C. §4501 et seq. |
| 105. |
Full text available at https://www.congress.gov/bill/116th-congress/house-bill/748?q=%7B%22search%22%3A%5B%22cite%3APL116-136%22%5D%7D&s=3&r=1. |
| 106. |
Individual bill text not available on Congress.gov as of April 3, 2020. For bill details see U.S. House Committee on Financial Services, "Committee Democrats at Work: Rep. Vargas Introduces Bill to Provide Additional Defense Production Act Funding During COVID-19 Crisis. |
| 107. |
Individual bill text not available on Congress.gov as of April 3, 2020. Draft text available at Office of U.S. Congressman French Hill, "Hill Responds to Public Health Emergency, Introduces Legislation to Increase U.S. Production of Medical Supplies," press release, March 30, 2020, https://hill.house.gov/news/documentsingle.aspx?DocumentID=6862. |
| 108. |
Individual bill text not available on Congress.gov as of April 3, 2020. For bill details see Office of Senator Chris Murphy, "Murphy, Schatz Announce Legislation Requiring Federal Government To Take Over Medical Supply Chain," press release, March 23, 2020, https://www.murphy.senate.gov/newsroom/press-releases/murphy-schatz-announce-legislation-requiring-federal-government-to-take-over-medical-supply-chain. |
| 109. |
Full text available at https://www.congress.gov/bill/116th-congress/senate-resolution/547. |
| 110. |
Individual bill text not available on Congress.gov as of April 3, 2020. Draft text available at Office of Senator Tammy Baldwin, "U.S. Senator Tammy Baldwin Announces New Legislation To Scale Up Made In America Production of Medical Supplies and Personal Protective Equipment for Hospitals and Health Care Workers," press release, March 22, 2020, https://www.baldwin.senate.gov/press-releases/scale-up-production-of-medical-supplies-and-ppe. |
| 111. |
Full text available at https://www.congress.gov/bill/116th-congress/house-resolution/906. |
| 112. |
Individual bill text not available on Congress.gov as of April 3, 2020. Draft text available at Office of U.S. Congressman Jamie Raskin, "Raskin Sponsors Legislation to Mobilize Production of Medical Supplies Under Defense Production Act," press release, March 26, 2020, https://raskin.house.gov/media/press-releases/raskin-sponsors-legislation-mobilize-production-medical-supplies-under-defense; a similar bill, H.R. 6410, was introduced on March 27, 2020. Full text for H.R. 6410 is not available on Congress.gov as of April 3, 2020. |
| 113. |
Individual bill text not available on Congress.gov as of April 3, 2020. Details available at "Office of Representative Tim Ryan, "Reps. Tim Ryan & Elissa Slotkin Announce Legislation Requiring Federal Government to Take Over Medical Supply Chain," press release, March 24, 2020, https://timryan.house.gov/media/press-releases/reps-tim-ryan-elissa-slotkin-announce-legislation-requiring-federal-government. |