Advanced Gene Editing: CRISPR-Cas9
Scientists have long sought the ability to control and modify DNA—the code of life. A gene editing technology known as CRISPR-Cas9 offers the potential for substantial improvement over other gene editing technologies in that it is simple to use and inexpensive and has a relatively high degree of precision and efficiency. These characteristics have led many in the scientific and business communities to assert that CRISPR-Cas9 will lead to groundbreaking advances in many fields, including agriculture, energy, ecosystem conservation, and the investigation, prevention, and treatment of diseases.
Over the next 5 to 10 years, the National Academy of Sciences projects a rapid increase in the scale, scope, complexity, and development rate of biotechnology products, many enabled by CRISPR-Cas9. Concomitant with the promise of potential benefits, such advances may pose new risks and raise ethical concerns. For example, a Chinese researcher recently claimed that he had created the first genetically engineered human babies. According to the researcher, he used CRISPR-Cas9 to disable a gene that will make it harder for the twin girls, who were born in November 2018, to contract human immunodeficiency virus (HIV). The as yet unsubstantiated claim has sparked outrage and ethical debates by the international scientific community and others. Prior use of CRISPR-Cas9 gene editing in human embryos was generally limited to non-viable embryos, in part, to address ethical concerns such as the fact that the genetic change would affect not only the immediate patient, but also future generations who would inherit the change.
Additionally, CRISPR-related approaches (gene drives) are being considered to reduce or eliminate the mosquito that serves as the primary vector for the transmission of Zika or malaria, thereby improving public health. Some scientists and environmental groups have raised ethical questions and expressed concerns about the unintended ecological consequences of eliminating a species or introducing a genetically modified organism into an open environment.
Some experts assert that the current system for regulating biotechnology products—the Coordinated Framework for the Regulation of Biotechnology—may be inadequate, with the potential to leave gaps in oversight. Regulatory gaps may lead to increased uncertainty that could affect the development of future biotechnology products or a loss of public confidence in the ability of regulators to ensure that such products are safe.
In the 116th Congress, policymakers may want to examine the potential benefits and risks associated with the use of CRISPR-Cas9 gene editing, including the ethical, social, and legal implications of CRISPR-related biotechnology products. Congress also may have a role to play with respect to regulation, research and development, and economic competitiveness associated with CRISPR-Cas9 gene editing and future biotechnology products.
Advanced Gene Editing: CRISPR-Cas9
Jump to Main Text of Report
Contents
- Introduction
- Overview
- What Is CRISPR-Cas9?
- Gene Editing
- How CRISPR-Cas9 Technology Works
- What Are Gene Drives?
- CRISPR-Cas9 Market Projections, Investments, and R&D Spending
- Market Projections
- Private Investments
- Federal R&D Funding and Scientific Publications
- The Coordinated Framework for the Regulation of Biotechnology
- Application Areas and Issues for Consideration
- Human Health and Medicine
- Diabetes
- Malaria
- Sickle Cell Disease
- Duchenne Muscular Dystrophy
- Antibiotic Resistance
- Biomedical and Clinical Research: Heritable Versus Non-Heritable Changes
- U.S. Regulation and Oversight of Biomedical and Clinical Research
- Ethical Considerations
- Agricultural Development
- U.S. Regulation and Oversight of Agricultural Biotechnology
- Social Acceptance and Ethical Concerns
- CRISPR-Cas9 and International Agriculture
- Industrial Biotechnology
- Ecosystem Management and Conservation
- Gene Drives and Environmental Concerns
- U.S. Regulation and Oversight of Gene Drives
- Social Acceptance and Ethical Concerns
- International Regulation of Genetically Modified Organisms
- Basic Research
- National Security Concerns
Summary
Scientists have long sought the ability to control and modify DNA—the code of life. A gene editing technology known as CRISPR-Cas9 offers the potential for substantial improvement over other gene editing technologies in that it is simple to use and inexpensive and has a relatively high degree of precision and efficiency. These characteristics have led many in the scientific and business communities to assert that CRISPR-Cas9 will lead to groundbreaking advances in many fields, including agriculture, energy, ecosystem conservation, and the investigation, prevention, and treatment of diseases.
Over the next 5 to 10 years, the National Academy of Sciences projects a rapid increase in the scale, scope, complexity, and development rate of biotechnology products, many enabled by CRISPR-Cas9. Concomitant with the promise of potential benefits, such advances may pose new risks and raise ethical concerns. For example, a Chinese researcher recently claimed that he had created the first genetically engineered human babies. According to the researcher, he used CRISPR-Cas9 to disable a gene that will make it harder for the twin girls, who were born in November 2018, to contract human immunodeficiency virus (HIV). The as yet unsubstantiated claim has sparked outrage and ethical debates by the international scientific community and others. Prior use of CRISPR-Cas9 gene editing in human embryos was generally limited to non-viable embryos, in part, to address ethical concerns such as the fact that the genetic change would affect not only the immediate patient, but also future generations who would inherit the change.
Additionally, CRISPR-related approaches (gene drives) are being considered to reduce or eliminate the mosquito that serves as the primary vector for the transmission of Zika or malaria, thereby improving public health. Some scientists and environmental groups have raised ethical questions and expressed concerns about the unintended ecological consequences of eliminating a species or introducing a genetically modified organism into an open environment.
Some experts assert that the current system for regulating biotechnology products—the Coordinated Framework for the Regulation of Biotechnology—may be inadequate, with the potential to leave gaps in oversight. Regulatory gaps may lead to increased uncertainty that could affect the development of future biotechnology products or a loss of public confidence in the ability of regulators to ensure that such products are safe.
In the 116th Congress, policymakers may want to examine the potential benefits and risks associated with the use of CRISPR-Cas9 gene editing, including the ethical, social, and legal implications of CRISPR-related biotechnology products. Congress also may have a role to play with respect to regulation, research and development, and economic competitiveness associated with CRISPR-Cas9 gene editing and future biotechnology products.
Introduction
Genes, the fundamental code of life, are written in DNA (deoxyribonucleic acid). Before DNA was even discovered, humans sought to manipulate genes through selective breeding. Since its discovery, scientists, science fiction writers, philosophers, and others have speculated on the implications of being able to modify DNA. Over the last half century, billions of dollars and immeasurable effort have been devoted to understanding, characterizing, and controlling DNA. This report describes a gene editing technology, known as CRISPR-Cas9, with the potential to revolutionize genetic engineering and the biotechnology industry. The report then provides information on the potential economic benefits of the technology and identifies some issues for congressional consideration, including the regulation of current and future products, national security concerns, and ethical and societal issues surrounding the use of the technology.
What Is CRISPR-Cas9?
CRISPR-Cas9 is a gene editing technology that offers the potential for substantial improvement over other gene editing technologies1 in ease of use, speed, efficacy, and cost. These characteristics led Science magazine to name CRISPR-Cas9 gene editing technology "Breakthrough of the Year" in 2015.2 Many in the scientific, engineering, and business communities believe that CRISPR-Cas9 may offer revolutionary advances in the investigation, prevention, and treatment of diseases; understanding of gene function; improving crop yields and developing new varieties; production of chemicals used in biofuels, adhesives, and fragrances; and control of invasive species.3
CRISPR is an acronym for "clustered regularly interspaced short palindromic repeats," which are unique DNA sequences found in some bacteria and other microorganisms. These sequences, along with the genes that are located next to them, known as CRISPR-associated or Cas genes, form an immune system that protects against viruses and other infectious DNA. The CRISPR system identifies, cuts, and destroys foreign DNA. Researchers have identified five different types of CRISPR systems. The most studied CRISPR system is associated with the Cas9 protein and is known as CRISPR-Cas9. During 2012 and 2013, researchers modified CRISPR-Cas9 to serve as an effective and efficient technology for editing the genomes4 of plants, animals, and microorganisms. Since then, CRISPR-Cas9 has been used to modify the genomes of a variety of species—ranging from mice and fruit flies to corn and yeast. Many in the scientific community believe CRISPR-Cas9 has shifted the paradigm with its simplicity and low cost relative to other methods of gene editing—removing barriers to widespread adoption and creating new research opportunities.5 This report focuses on the use of CRISPR-Cas9 as a gene editing technology, which is sometimes referred to as CRISPR in the report. However, other CRISPR systems are currently in development and use.6
Despite this promise, technical challenges to realizing the full potential of CRISPR-Cas9 remain. Researchers largely agree that efficiently delivering the technology to particular cells, tissues, or organs, and reducing off-target activity (i.e., the number of unintended genetic changes) are among the most pressing challenges. Off-target activity may increase the risk of cancer, and thus improved delivery and specificity are especially important for the development of gene therapy applications.7 Scientists are investigating ways to overcome these challenges and improve CRISPR-Cas9.
Gene Editing
For decades, scientists have altered genes using radiation or chemicals. These methods produce unpredictable results. The invention of recombinant DNA technology in the 1970s allowed scientists to insert new DNA into genes in a directed way, but inserting a specific gene or sequence within the genome remained technically challenging and imprecise.
Gene editing is a newer technique that is used to make specific and intentional changes to DNA.8 Gene editing can be used to insert, remove, or modify DNA in a genome. All gene editing technologies involve an enzyme known as a nuclease for cutting the DNA, in addition to a targeting mechanism that guides the enzyme to a specific location on the DNA strand (i.e., a gene within the genome). Gene editing has traditionally involved the insertion, removal, or modification of a single gene, but with CRISPR-Cas9 multiple genes can be targeted simultaneously. Such multi-gene editing is generally referred to as genome editing.
How CRISPR-Cas9 Technology Works
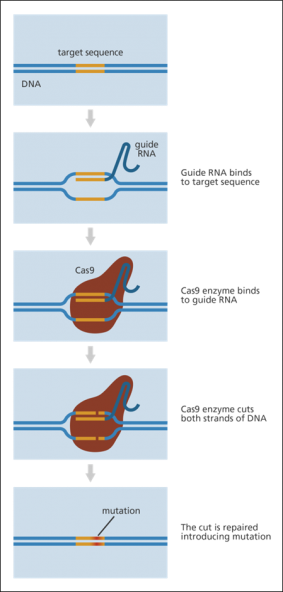
CRISPR-Cas9 is a gene editing technology that uses a combination of (1) an enzyme that cuts DNA (Cas9, a nuclease) and (2) a guiding piece of genetic material (guide RNA) to specify the location in the genome. Generally, the guide RNA targets and binds to a specific DNA sequence, and the attached Cas9 enzyme cleaves both strands of DNA at that site. This cut can be used to insert, remove, or edit the DNA sequence. The cut is then repaired and the changes incorporated (Figure 1). This specificity of modification is one feature that differentiates CRISPR-Cas9 from predecessor genome editing systems.
Scientists can create a guide RNA corresponding to almost any sequence within an organism's genome. This flexibility allows for the potential application of the technique to a very wide range of genomes, including microorganisms, animals, or plants. If the sequence of the desired target or gene (and its function) is known, in theory, CRISPR-Cas9 could be used to alter the function of a cell or organism.
The basic CRISPR-Cas9 technology, specifically the Cas9 nuclease, has also been adapted by researchers to allow for additional modifications to the genome beyond the cutting of both strands of the DNA. For example, researchers have adapted Cas9 so that it can be used to change a single base9 in a gene (base editing), cut a single strand of DNA, or activate or repress the expression of a gene (i.e., increase or decrease the production of a molecule, typically a protein).10
 |
|
Source: "What Is CRISPR-Cas9?," at http://www.yourgenome.org/facts/what-is-crispr-cas9. Note: Image credit: Genome Research Limited. |
What Are Gene Drives?
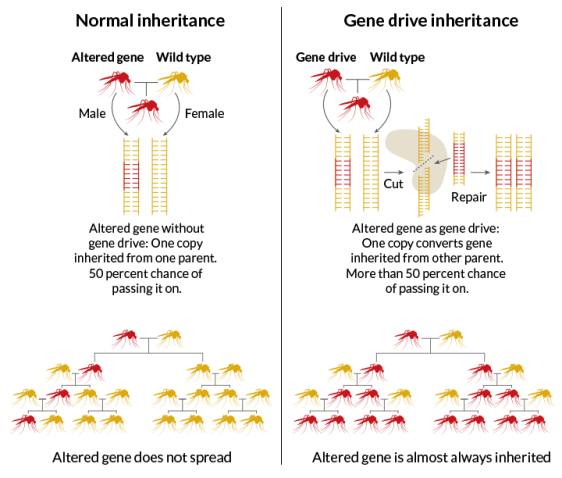
CRISPR-Cas9 has led to recent breakthroughs in gene drive research. A gene drive is a system of biasing inheritance to increase the likelihood of passing on a modified gene. Offspring inherit one copy of each gene from its parents. Normally, this limits the total incidence of mutations over generations (Figure 2). Gene drive components cause the modified DNA to copy itself into the DNA from the unmodified parent. The result is the preferential increase in a specific trait from one generation to the next and, in time, possibly throughout the population. CRISPR-Cas9 has allowed researchers to more effectively insert a modified gene and the gene drive components. Gene drives have been suggested as a way to eliminate or reduce the transmission of disease, eradicate invasive species, or reverse pesticide resistance in agriculture. The self-propagating nature of gene drives is also accompanied by concerns (described later in the report).
CRISPR-Cas9 Market Projections, Investments, and R&D Spending
CRISPR-Cas9 technology is still in its infancy, with many of the hoped-for applications potentially years in the future. However, the interest, efforts, and investments of the industrial and financial communities suggest the potential economic and other societal benefits are substantial. Among the early indicators of the potential value of CRISPR-enabled products are fees being paid to license CRISPR patents, investments in firms with potential interests in CRISPR intellectual property, the type of companies investing in CRISPR research, and early applications. This section discusses recent projections made by market research firms, select private investments, federal research and development funding, and statistics on scientific publications.
 |
|
Source: https://www.sciencenews.org/article/gene-drives-spread-their-wings. |
Market Projections
A number of research firms have published market projections for gene editing, including CRISPR-Cas9 and other technologies. Application areas include human therapeutics, research tools, crops, livestock, yogurts, cheeses, and more.
- In August 2018, Ireland-based Research and Markets estimated that the global market for gene editing will grow at a compound annual growth rate (CAGR) of 33.26% from $551.2 million in 2017 to $3.087 billion in 2023.11 An earlier report projected that the North American market will account for the largest share of the gene editing market due to "increasing awareness of technology, proximity of companies, and early adoption of latest treatments." Asia was expected to be the second largest market, due to "increasing government funding of research, economic prosperity, early adoption of latest technology and the relaxed regulatory environment." The European market was projected to be the third largest market, hampered by "the stringent regulatory environment and slow growth due to the economic crisis."12
- India-based Markets and Markets estimated that the global market for gene editing will increase from $3.19 billion in 2017 to $6.28 billion in 2022, a CAGR of 14.5%. CRISPR technology was expected to be the largest and fastest-growing segment of this market in 2017.13
- Zion Market Research estimated that the CRISPR gene editing market in 2017 was $477 million and projected that it will reach $4.271 billion by 2024, a CAGR of 36.8%.14
- A February 2017 projection by the U.S.-based market research firm Grand View Research anticipates the global market for gene editing will reach $8.1 billion by 2025.15
Private Investments
Private investments are a commonly used metric for assessing the economic potential of a technology. Investments are being made by and in companies of varying size and technology maturity that are conducting CRISPR research. In addition, these companies are engaging in a wide range of partnerships. Here are several examples of recent investments in CRISPR-focused gene editing firms:
- Editas Medicine (headquartered in Cambridge, MA) raised approximately $97.5 million in its February 2016 initial public offering. In follow-on offerings in March and December 2017, Editas raised approximately $96.7 million and $57.2 million, respectively. In January 2018, the company completed at-the-market offerings and received net proceeds of approximately $48.5 million.16 The firm has licensed CRISPR and other gene editing patent rights from the Broad Institute, the Massachusetts Institute of Technology (MIT), Harvard University, and others.17 As of November 15, 2018, the company's market capitalization was $1.34 billion.18 In March 2017, Editas reportedly entered into an agreement with Irish pharmaceutical company Allergan under which Editas was to receive a $90 million up-front payment for an option to license up to five preclinical programs targeting eye disease.19 Editas has also partnered with Juno Therapeutics for cancer-related research using CRISPR; under the terms of the agreement, Juno was to pay Editas an initial payment of $25 million and up to $22 million in research support for three programs over five years.20 Editas has also engaged in a three-year research and development (R&D) collaboration deal with San Raffaele Telethon Institute for Gene Therapy to research and develop next-generation stem cell and T-cell therapies for the treatment of rare diseases.21
- CRISPR Therapeutics AG (headquartered in Basel, Switzerland, with R&D operations in Cambridge, MA), a firm founded by early CRISPR pioneer Emmanuelle Charpentier, has raised a total of almost $140 million, including a $38 million B-series round of financing in June 2016.22 The company raised an additional $56 million in its October 2016 initial public offering, followed by $122.6 million in January 2018 and $187.6 million in September 2018 in subsequent offerings. In addition, in August 2016, CRISPR Therapeutics and pharmaceutical company Bayer AG founded Casebia Therapeutics, a joint research venture "to discover, develop and commercialize new breakthrough therapeutics to cure blood disorders, blindness, and congenital heart disease." Bayer stated that it will be providing at least $300 million for R&D by the joint venture and that it had taken a $35 million equity stake in CRISPR Therapeutics.23 CRISPR Therapeutics also has collaboration and joint development agreements with Boston-based Vertex Pharmaceuticals to use CRISPR-Cas9 to discover and develop potential new treatments aimed at the underlying genetic causes of human disease. CRISPR Therapeutics and Vertex have reportedly launched the first in-human clinical trial of CRISPR genome editing technology sponsored by U.S. companies. The trial is testing an experimental therapy for the blood disorder β-thalassemia in Regensburg, Germany. As of November 15, 2018, the company's market capitalization was $1.89 billion.24
- Caribou Biosciences, Inc. (headquartered in Berkeley, CA), a firm founded by Jennifer Doudna and other scientists from the University of California, Berkeley, based on an exclusive license to the CRISPR work of that university and the University of Vienna, raised $30 million in private financing in May 2016.25
|
Broad Institute's Restrictions on CRISPR Licensing The Broad Institute prohibits the use of the licensed CRISPR technology for
|
Examples of other efforts focused on CRISPR technology and the development, application, and commercialization of CRISPR-enabled products include the following:
- The Parker Institute for Cancer Immunotherapy, a non-profit organization formed in April 2016 with a $250 million grant from the Parker Foundation, agreed to sponsor the first in-human clinical trials of CRISPR-enabled technology targeting three types of cancer. The trial, led by the University of Pennsylvania, will use CRISPR-modified T-cells, a part of the human immune system, to treat myeloma, melanoma, and sarcoma. The trial has been approved by the National Institutes of Health's Recombinant DNA Advisory Committee and is awaiting approval from review boards at the centers where the trials are to be held as well as the Food and Drug Administration. The trial was initially expected to commence in 2017, but is still awaiting final approval.26
- In September 2016, agrochemical and agricultural biotechnology corporation Monsanto secured a worldwide non-exclusive license agreement for agricultural applications of CRISPR technology from the Broad Institute.27 With respect to its intended uses, Monsanto stated, "Genome-editing technology is complementary to our ongoing discovery research and provides an incredible resource to further unlock our world-leading germplasm and genome libraries."28
- Calyxt, Inc. (formerly Cellectis Plant Sciences, Inc., headquartered in New Brighton, MN), has exclusive rights to a group of patents owned by the University of Minnesota for engineering plant genomes with a focus on products such as low trans-fat soybean oil, cold storable potato, gluten reduced wheat, and low saturated canola oil for the food and agriculture industries.29
Federal R&D Funding and Scientific Publications
The potential of CRISPR-Cas9 gene editing is further reflected in the rapid increase in CRISPR-related federal research funding and scientific publications. As shown in Table 1, NIH funding for CRISPR-related research grew from more than $5 million in FY2011 to $1.1 billion in FY2018. Similarly, the number of CRISPR-related scientific publications increased from 87 in 2011 to 3,917 in 2018 (Table 2).
|
Fiscal Year |
Projects |
Total Funding |
|
2011 |
7 |
$5,070,129 |
|
2012 |
9 |
$7,432,520 |
|
2013 |
30 |
$12,505,507 |
|
2014 |
161 |
$85,298,742 |
|
2015 |
551 |
$267,055,410 |
|
2016 |
1,245 |
$603,205,999 |
|
2017 |
2,031 |
$947,465,783 |
|
2018 |
2,651 |
$1,155,385,840 |
|
Total |
6,685 |
$3,083,419,930 |
Source: CRS analysis of data from NIH RePorter (https://projectreporter.nih.gov/reporter.cfm) as of November 20, 2018.
|
Year |
Publications |
|
2011 |
87 |
|
2012 |
137 |
|
2013 |
300 |
|
2014 |
670 |
|
2015 |
1,457 |
|
2016 |
2,594 |
|
2017 |
3,738 |
|
2018 |
3,917 |
|
Total |
12,900 |
Source: CRS analysis of data on scientific publications from Scopus (https://www.scopus.com) as of November 20, 2018.
The Coordinated Framework for the Regulation of Biotechnology
The fundamental federal guidance for regulating biotechnology products, including those developed using CRISPR-Cas9, is the Coordinated Framework for the Regulation of Biotechnology (the Coordinated Framework) originally published in 1986 by the White House Office of Science and Technology Policy (OSTP).30 A key principle in this regulatory structure is that genetically engineered products should continue to be regulated according to their characteristics and unique features, not their production method—that is, whether or not they were created through genetic engineering techniques (e.g., CRISPR-Cas9, ZFNs, and TALENs). The framework provides a regulatory approach intended to ensure the safety of biotechnology research and products, using existing statutory authority and previous agency experience. The Coordinated Framework consists of three primary agencies—the Environmental Protection Agency (EPA), the U.S. Department of Agriculture (USDA), and the Food and Drug Administration (FDA).
- EPA protects human health and the environment by regulating genetically engineered products that qualify as pesticides under the Federal Insecticide, Fungicide, and Rodenticide Act (7 U.S.C. §136 et seq.); it sets guidelines on the amount of pesticidal residue that may be present in food under section 408 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. §301 et seq.); and it regulates new chemical substances derived from microbial biotechnology under the Toxic Substances Control Act (15 U.S.C. §2601 et seq.).
- USDA regulates biotechnology products that may pose a risk to agricultural plant and animal health under the Plant Protection Act (7 U.S.C. §7701 et seq.) and the Animal Health Protection Act (7 U.S.C. §8301 et seq.).
- FDA protects human health and safety by regulating human and animal drugs, human and animal foods derived from genetically engineered plants, and genetically engineered animals under the authorities of the Federal Food, Drug, and Cosmetic Act and the Public Health Service Act (42 U.S.C. §201 et seq.).
New biotechnology developments, continuing opposition by consumer groups and environmentalists, and perceived inadequacies of federal regulation led the Obama Administration to issue a memorandum on July 2, 2015, to update the Coordinated Framework to ensure that the regulatory structure is capable of meeting future biotechnology risks.31
The memorandum observed that each of the federal agencies regulating biotechnology had developed its own regulations and guidance documents to implement its authority under current statutes, resulting in "a complex system for assessing and managing health and environmental risks of the products of biotechnology." Since a 1992 update, advances in science and technology have "dramatically altered the biotechnology landscape," according to the memorandum. CRISPR-Cas9 and other gene-editing systems were unknown when the Coordinated Framework was published in 1986, or at the time of the 1992 update.32 The White House memorandum stated that a new update to the Coordinated Framework was needed to "facilitate the appropriate federal oversight by the regulatory system and increase transparency while continuing to provide a framework for advancing innovation."
The White House memorandum initiated a process to achieve the following objectives: (1) update the Coordinated Framework to clarify the agencies' roles and responsibilities to regulate biotechnology products; (2) formulate a long-term strategy to ensure that the regulatory system can adequately assess any risks associated with future products of biotechnology while "increasing transparency and predictability and reducing unnecessary costs and burdens"; and (3) commission an external, independent analysis of the future landscape of biotechnology products.
The White House memorandum established a Biotechnology Working Group (BWG) under the Emerging Technologies Interagency Policy Coordination Committee. The working group included representatives of the White House, EPA, FDA, and USDA. The update to the Coordinated Framework by the three primary regulatory agencies overseeing biotechnology was published in January 2017 following three public comment sessions.33 The 2017 update discussed the roles of the three agencies and the coordination of oversight responsibilities. The update generally concluded that the existing structure of regulation among the three agencies remained sound with respect to protecting health and the environment. However, the update did note that uncertainty with respect to agency jurisdiction, and a lack of predictability of timeframes for review, imposed costs on small and mid-size companies and academe. In reinforcing the logic of the 1986 Coordinated Framework, the update also explicitly stated that the "specific regulatory path (and relevant procedures) applicable to any product, including a biotechnology product, is dependent on the nature and characteristics of the product and its application."34
To achieve the second objective of proposing a long-term strategy for biotechnology product regulation, the BWG published the National Strategy for Modernizing the Regulatory System for Biotechnology Products in September 2016.35 The goal of the proposed national strategy is to ensure that the regulatory agencies can "efficiently assess risks of future biotechnology products while supporting innovation, protecting health and the environment, promoting public confidence in the regulatory process, increasing transparency and predictability, and reducing unnecessary costs and burdens."
To assess the future landscape of biotechnology products, EPA, FDA, and USDA commissioned a study in early 2016 by the National Academy of Sciences (NAS) to identify (1) major advances and potential new types of biotechnology products over the next 5 to 10 years, (2) potential future products that might pose a different type of risk relative to existing products and organisms, (3) areas in which the risks or lack of risk relating to biotechnology are well understood, and (4) the scientific capabilities, tools, and expertise that may be useful to the regulatory agencies as they oversee potential future products of biotechnology. The NAS published its final report in February 2017, emphasizing that the new products stemming from genomic research could overwhelm the three lead regulatory agencies, and outlining a strategic approach to risk management and coordination among these regulatory agencies.36
Despite recent efforts to update the Coordinated Framework, CRISPR-Cas9 technology and other gene-editing systems raise substantive questions about how (or whether) the products resulting from these technologies are to be regulated, and if so, under what statutory authorities. Specifically, the 2017 NAS report found that "regulators will face difficult challenges as they grapple with a broad array of new types of biotechnology products—for example, cosmetics, toys, pets, and office supplies—that go beyond contained industrial uses and traditional environmental release."37 Some of the products that are likely to be developed using CRISPR-Cas9 will not fit neatly into the established categories that regulatory agencies worldwide have developed over the past 30 years. Potential issues for consideration when developing regulations for biotechnology products developed using CRISPR-Cas9 are discussed in more detail later.
Application Areas and Issues for Consideration
The following sections provide examples of the current and potential uses of CRISPR-Cas9 across a broad set of areas. Some sections include a description of issues for congressional consideration, such as the regulation of future biotechnology products, international implications, and societal, ethical, environmental, and national security concerns.
Human Health and Medicine
Many experts assert that CRISPR-Cas9 may offer the means to prevent, treat, or cure medical conditions or disease producing substantial savings in direct and indirect economic costs, in addition to reducing the toll from pain, debilitation, and death. The following applications are intended to be exemplary, not comprehensive.
Diabetes
The California Institute for Regenerative Medicine (CIRM) awarded a grant to researchers at Children's Hospital Los Angeles who are using CRISPR-Cas9 to develop a personalized approach for treating genetic forms of diabetes (e.g., Type I diabetes) by replacing insulin-producing cells in patients. The approach is expected to be an improvement over existing methods of treating Type I diabetes. By using the patient's own cells the risk of transplant rejection is reduced and patients would not be reliant on the limited availability of outside donors. Researchers believe that the approach may also eventually offer treatment for non-autoimmune diabetes (such as Type II).38 According to the American Diabetes Association, the disease affects nearly 30 million Americans. The association estimates the total economic cost of diagnosed diabetes in the United States in 2012 at $245 billion, including $176 billion in direct medical costs and $69 billion in reduced productivity.39
Malaria
A variety of CRISPR-enabled approaches are being considered in efforts to reduce or eliminate malaria, one of the most widespread and lethal illnesses in the world. Effective modification, reduction, or elimination of the Anopheles mosquito—the primary vector for the transmission of malaria—could substantially reduce these costs and open up new economic opportunities in many of the world's poorest nations. CRISPR-enabled approaches include the use of gene drives, a genetic tool that results in a modified gene being preferentially passed to offspring. This might offer a means by which all Anopheles mosquitos could be made infertile40 or that would result in all offspring being male.41 If successful, these approaches would, in time, drastically reduce or even possibly eliminate the population being targeted. Another CRISPR-enabled approach seeks to make the Anopheles mosquito resistant to the malaria parasite.42
Fighting malaria is a top priority of the Bill and Melinda Gates Foundation, which among other efforts, is investing heavily in the development of CRISPR-based gene drive technologies to reduce or eradicate the Anopheles mosquito in sub-Saharan Africa. For example, the Bill and Melinda Gates Foundation has awarded approximately $75 million to Target Malaria—a non-profit research consortium—whose work is focused on reducing the number of female mosquitos in three closely related Anopheles species that are responsible for most of the malaria transmission in Africa.43 According to Roll Back Malaria,44 the disease may account for as much as 40% of public health expenditures in some countries.45 According to the Centers for Disease Control and Prevention, the direct costs of malaria (e.g., illness, treatment, and premature death) have been estimated to be at least $12 billion per year globally, and the cost in lost economic growth is much greater.46
Similar approaches are being discussed for reducing the transmission of other mosquito-borne viral diseases including, Zika, dengue fever, yellow fever, West Nile, and St. Louis encephalitis.47
Sickle Cell Disease
In October 2018, the U.S. Food and Drug Administration (FDA) accepted the application of two biotechnology companies—CRISPR Therapeutics and Vertex—for an experimental gene therapy treatment for sickle cell disease (SCD). The treatment would consist of using CRISPR-Cas9 to modify stem cells that are isolated from a patient's blood and then reinfused to produce high levels of fetal hemoglobin. The higher levels of fetal hemoglobin are expected to counteract severe pain caused by the sickle cell mutation.48 SCD affects approximately 100,000 Americans. According to a 2009 study, the total estimated annual U.S. cost of medical care for SCD exceeded $1.1 billion.49
Duchenne Muscular Dystrophy
Researchers at the University of Texas Southwestern Medical Center have demonstrated the ability to use CRISPR-Cas9 to make genetic repairs in cells that allows them to produce dystrophin.50 Dystrophin is a protein that patients with Duchenne Muscular Dystrophy (DMD),51 a genetic disorder, cannot produce. The absence of dystrophin cripples those with DMD, and generally leads to heart and respiratory muscle problems, and death. According to a comprehensive cost-of-illness study sponsored by the Muscular Dystrophy Association, the annual U.S. costs for DMD are estimated at $362-$488 million per year, about $51,000 per year per patient in medical expenses, nonmedical costs, and lost income.52
Antibiotic Resistance
CRISPR-Cas9 holds promise in combating antibiotic resistant pathogens.53 According to the Centers for Disease Control and Prevention, approximately 2 million people are infected annually with bacteria that are resistant to antibiotics and at least 23,000 people die each year as a result of such infections.54 CRISPR-Cas9 has been shown to effectively target and eliminate bacterial species, including antibiotic resistant strains, from a community of bacteria. This precise targeting allows the elimination of harmful bacteria, but avoids beneficial bacteria (e.g., bacteria that aid in digestion). Additionally, unlike traditional antibiotics it would be difficult for bacteria to develop resistance to CRISPR-based antimicrobials because such a resistance would likely destroy the bacteria's defense mechanisms to viruses. According to researchers, the largest obstacle to development of CRISPR-based antimicrobials is identifying an effective delivery route.55
Biomedical and Clinical Research: Heritable Versus Non-Heritable Changes
Possible clinical and biomedical applications of gene editing with CRISPR-Cas9 are numerous, as noted above, and would include, among others, modification of genes in specific individuals to treat or possibly cure disease. Such a technique could also potentially be used to modify very early human embryos or gametes (eggs and sperm) to alter deleterious genes. In this case, changes made to the genetic material would be in the germline, and therefore, changes would be retained and passed on to future generations.56 In contrast, changes made to genetic material in other cells in the body (called somatic cells) would affect only the individual in which they were made, and would not be passed on to future offspring. This distinction—using gene editing for somatic (non-heritable) versus germline (heritable) genetic modification—is significant from an ethical and societal standpoint. This distinction is reflected in the regulatory paradigm for regulating all gene editing research, including CRISPR-Cas9, and has been relevant in discussions of all gene editing, engineering, or modification techniques that might theoretically be applied to human embryos.
Progress toward carrying out clinical trials using CRISPR-Cas9 for non-heritable genetic modification is currently being made in multiple countries. China has been leading research efforts in this area, with researchers at Sichuan University carrying out the first-ever human trial of CRISPR-Cas9 in late 2016 as part of a broader clinical trial.57 This study, treating a total of ten advanced lung cancer patients with CRISPR-Cas9 -modified immune cells (T-cells), was primarily a study of safety and not efficacy, and was planned to monitor patients for a total of six months for adverse effects of the treatment.58 Since that time, researchers in China have initiated several additional clinical trials, and China continues to be in the forefront of this research. In the United States, two clinical trials (one a Phase 1 trial, the other a Phase 1/2 trial59) are underway, one targeting cancer, the other sickle cell disease (SCD). At the University of Pennsylvania, researchers have begun recruiting for a trial—similar to the 2016 trial by researchers at Sichuan University—whereby human T-cells will be modified using CRISPR-Cas9 and introduced into cancer patients. This trial received approval from the NIH's Recombinant DNA Advisory Committee (RAC)60 and is also small, including 18 patients, with a primary focus on safety.61 In addition, the FDA has recently lifted a clinical hold on an investigational new drug application (IND) submitted to the agency by Vertex Pharmaceuticals and CRISPR Therapeutics for a trial that will test CRISPR-modified cells in patients with SCD, allowing it to go forward. This study is now recruiting participants.62 Before clinical trials may begin in the United States, researchers must submit, and the FDA must accept, an IND outlining specific parameters of the research trial.63 The FDA also, on November 30, 2018, authorized a third trial sponsored by the U.S. company Editas Medicine that plans to use a CRISPR-based therapy to treat a rare genetic disease that causes blindness.64
CRISPR-Cas9 has also been used to make heritable modifications in both viable and non-viable human embryos in research being carried out in other countries, and, more recently, by researchers based in the United States. In May 2015, Chinese scientists were the first to use CRISPR-Cas9 in human embryos. These scientists published results of an experiment that attempted to modify the genetic make-up of non-viable human embryos using CRISPR-Cas9. This experiment attempted to modify a gene for beta-thalassemia, a fatal blood disorder.65 In April 2016, a second team of Chinese researchers published results of a study that used CRISPR-Cas9 to try to introduce a mutation that confers HIV-resistance into non-viable human embryos.66 Neither of these studies demonstrated a high success rate, nor an ability to precisely direct editing of the host genome.
In August 2017, an international team led by researchers at Oregon Health and Science University (OHSU) reported using CRISPR in viable human embryos to correct a genetic defect which causes hypertrophic cardiomyopathy (HCM), a leading cause of sudden death in young athletes.67 The experiment reportedly showed that 72.4% of the modified embryos ended up with the healthy version of the relevant gene (vs. the expected 50% without the use of CRISPR). This research has subsequently generated debate in the scientific community, as the results—and specifically the claim that the embryo preferentially used self-directed as opposed to template-directed repair to replace the faulty gene—were called into question by some in the scientific community.68 Although the researchers did further testing of the embryos to resolve some of the questions, the study has yet to be replicated, and reproduction of this type of study will likely be slower due to the restrictions on federal funding of research in human embryos. Earlier in 2017, Chinese scientists had published results of a study using CRISPR-Cas9 also in viable human embryos, again targeting a gene for beta-thalassemia.69 Next-generation versions of CRISPR are being developed, and one of those—base editing—was recently used in a Chinese study in viable human embryos to correct a gene associated with Marfan Syndrome.70 The results of this study were more successful and demonstrated a repair rate of 89% with no off-target effects detected.
|
Chinese Scientist First to Modify Humans? On November 26, 2018, Chinese scientist He Jiankui announced the birth of twin girls who had their DNA edited using CRISPR. The claims have not been published in the peer reviewed literature and therefore cannot be verified; however, the announcement has generated significant backlash amongst both Chinese and American scientists involved in this type of research, as well as from the Chinese government and the National Institutes of Health. Sources: NPR, "Chinese Scientist Says He's First to Create Genetically Modified Babies Using CRISPR," https://www.npr.org/sections/health-shots/2018/11/26/670752865/chinese-scientist-says-hes-first-to-genetically-edit-babies; NIH, "Statement on Claim of First Gene-Edited Babies by Chinese Researcher," November 28, 2018, https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-claim-first-gene-edited-babies-chinese-researcher. |
Although the CRISPR-Cas9 platform was first described in 2012 when scientists at the University of California, Berkeley, published a study using the technique in vitro,71 its use in the initial 2015 Chinese study in non-viable human embryos reignited the traditional debate and highlighted concerns about engineering changes to the human germline. The concerns of scientists and others have been, to some extent, borne out by the recent unverified claim of the birth of children with CRISPR modified DNA. In addition, the initial Chinese study prompted discussion about how existing law and regulation in the United States would apply to the conduct of this type of research, its clinical testing in humans, and specifically its applications in human embryos. With the 2017 privately-funded study on human embryos in the United States, the discussion around domestic regulation has intensified. Likely in response to some of these advances and discussions, several key developments have occurred recently in U.S. regulation of this type of research.
U.S. Regulation and Oversight of Biomedical and Clinical Research
One way that CRISPR-Cas9 technology triggers federal oversight is with respect to the conduct of certain biomedical and clinical research. The federal government, both as a funder of biomedical research and as a regulator of the safety and effectiveness of medical products used to treat disease, can impose requirements on research as a condition for receiving either federal funding or FDA premarket review of a new medical product (such as a drug, device, or biologic).
Regulation of clinical research pursuant to premarket requirements for a new medical product is the responsibility of the FDA. Federal oversight of government funding for biomedical research is generally the purview of NIH, as NIH is the predominant federal funder of this type of research.72 In addition, federal funding for biomedical research may be restricted, banned, or specifically directed by Congress through the annual appropriations process for these agencies.73 NIH is funded by the Departments of Labor, Health and Human Services, and Education, and Related Agencies (LHHS) appropriations bill, whereas FDA is funded by the Agriculture, Rural Development, Food and Drug Administration, and Related Agencies appropriations bill.
There are federal and congressional oversight mechanisms and regulations with respect to CRISPR-Cas9 research at FDA and NIH, and in the LHHS and Agriculture appropriations bills. As described in the following paragraphs, these mechanisms include requirements pursuant to the receipt of certain NIH funding; a LHHS appropriations rider limiting the use of federal funds for research on or involving human embryos; an appropriations provision limiting FDA's use of funding for review of certain embryo research using CRISPR-Cas9 and other gene editing technologies; and FDA regulatory requirements for certain clinical research for the eventual marketing of CRISPR-Cas9 applications.
NIH Guidelines for Research Involving Recombinant DNA and the Recombinant DNA Advisory Committee (RAC)
As stipulated by its policy, NIH will not fund any research using gene-editing technologies (including CRISPR-Cas9) in human embryos. The policy states that "[t]he concept of altering the human germline in embryos for clinical purposes has been debated over many years from many different perspectives, and has been viewed almost universally as a line that should not be crossed."74 NIH-funded research that uses CRISPR-Cas9—not in human embryos—has to comply with the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines) to receive and maintain funding. This also applies to non-NIH-funded CRISPR-Cas9 research carried out at institutions receiving NIH funding for other recombinant or synthetic nucleic acid research at the institution.75
For research that involves the transfer of recombinant nucleic acid molecules into human research participants, current NIH Guidelines require the research protocol to be registered, which may involve review by the NIH Recombinant DNA Advisory Committee (RAC). Per the NIH Guidelines, the RAC will not consider research proposals for germline modification. In addition, the research protocol must be approved by both the Institutional Review Board (IRB) and the Institutional Biosafety Committee (IBC).76
However, in August 2018, NIH published a notice in the Federal Register seeking comment on proposed changes to the NIH Guidelines. Specifically, NIH is proposing to eliminate the protocol registration and reporting requirements and also to eliminate the requirement for RAC review.77 The stated purpose of these changes is to streamline oversight of gene transfer clinical research trials, which many view as unnecessarily duplicative because this research has moved more into the mainstream and no longer presents unique safety concerns. NIH and FDA note in a recent article that "[i]n the view of the senior leaders of the FDA and NIH, there is no longer sufficient evidence to claim that the risks of gene therapy are entirely unique and unpredictable—or that the field still requires special oversight that falls outside our existing framework for ensuring safety."78 NIH also proposes to make changes to the oversight responsibilities of the IBCs so that their review of gene transfer research is no longer exceptional and instead is consistent with the review of other research protocols that come under the purview of the NIH Guidelines. NIH is proposing to maintain RAC as an advisory board to provide a forum for discussing and considering the ramifications of emerging biotechnologies, including synthetic biology, gene editing, and neurotechnology.79 While these proposed changes to the NIH Guidelines are being considered and finalized, NIH is not accepting submission of any new human gene transfer research protocols for registration, or any safety reports or amendments to existing human gene transfer research protocols.80
Dickey-Wicker Amendment to Labor-HHS-Education Appropriations
Since FY1996, a rider known as the Dickey-Wicker amendment has been attached to the Labor-HHS-Education appropriations bill each year in the annual appropriations process.81 This rider prohibits the Department of Health and Human Services (HHS) from using appropriated funds to support research in which human embryos are destroyed or in which human embryos are created for research purposes. The rider prohibits the NIH, or any other HHS agency, from using federal funds to support research involving human embryos, including the genetic modification of human embryos, and any modifications by CRISPR-Cas9. Because the FDA is funded through the annual Agriculture, Rural Development, Food and Drug Administration, and Related Agencies appropriations bill, this prohibition does not apply to the potential use of FDA funds to support activities related to research using human embryos.
Food and Drug Administration
Taking note of this new technology, Congress has acted to prevent FDA approval of medical products based on CRISPR-Cas9 and other gene editing technologies in human embryos. In the Consolidated Appropriations Act, 2018 (P.L. 115-141), Congress included a provision that prohibits the FDA from using appropriated funds to notify a sponsor or acknowledge receipt of a submission for an exemption for investigational use of a drug or biological product (i.e., an IND) for research in which a human embryo is created or modified to include a heritable genetic modification.82 This provision was first included in appropriations for FY2016 (the Consolidated Appropriations Act, 2016, P.L. 114-113).
FDA regulatory requirements apply to all clinical research, regardless of funding source that is carried out to investigate new, unapproved medical products such as drugs, devices, and biologics. Although FDA has not yet reviewed and approved or disapproved a CRISPR-Cas9 application, based on recent draft guidance documents published by FDA in July 2018, CRISPR-Cas9 products would meet the definition of a human gene therapy product and are biologics.83 In this case, clinical research with CRISPR-Cas9 products requires FDA acceptance of an IND; this regulatory requirement derives from FDA's authority to regulate biologics.84 Biologics must receive a license (i.e., authorization) from FDA prior to being marketed.85
Ethical Considerations
Somatic applications of CRISPR-Cas9 technology typically raise fewer ethical issues than do germline applications of the technology in humans. For some, the potential use of this technology in somatic cells for non-disease applications (also referred to as "enhancement") would raise ethical issues. An enhancement would be a modification to a normative non-disease trait to make an improvement to it; such traits might include, for example, strength or intelligence. Conversely, the modification of somatic cells using CRISPR-Cas9 for the purposes of treating or curing disease primarily raises issues of safety rather than of ethics. Specifically, with CRISPR-Cas9 gene editing, scientists are concerned, among other things, with the accuracy of the initial cut in the DNA; with the integration of the replacement genetic material being incorporated at the site of the cut; and with "off-target" activity (meaning unintended cuts and/or integration of replacement genetic material at additional unintended sites in the host genome). These problems have been shown to be fairly common in early research using CRISPR-Cas9. However, ethical issues could be raised secondary to safety concerns that were not adequately addressed prior to use of the technology in humans, and there may be differing perspectives on whether a safety concern has been adequately addressed, potentially compounding any ethical concerns.
Ethical considerations with respect to the use of CRIPSR-Cas9 arise predominantly with respect to the potential use of the technology to modify human embryos. However, ethical concerns about the genetic modification of the human germline are not new. Bioethicists, scientists, and others have debated the ethics of introducing changes to the human germline beginning with the advent of recombinant DNA technology and in the context of first human gene therapy and then human gene transfer research. Generally, the ethical concerns have centered on three main issues or variants of these issues:
- that the technology could create inherent inequities due to differential access by those with resources and those without;
- that changes to the germline would be passed on to future generations and therefore might alter the genetic makeup of the population in unintended or unforeseen ways and without the permission of those affected; and
- that modification might be used for enhancement purposes rather than only for curing or treating disease or restoring lost function.
Research that does not intend to modify human embryos, but rather that uses CRISPR-Cas9 to study genes involved in early development, may avoid some of these ethical quandaries. In addition, scientists have conducted some of the early research using CRISPR-Cas9 to modify non-viable embryos in an attempt to mitigate some of the ethical concerns with this type of research.
The publication of the first study using CRISPR-Cas9 in human embryos prompted the debate over germline modification to re-emerge in the scientific community, intensified by the perception that the new technology may be promising in ways not previously seen with respect to its ease of use and precision of editing. In response to this and other related research, members of the scientific community gathered at the International Summit on Human Gene Editing in December 2015—co-hosted by the National Academy of Sciences (NAS), the National Academy of Medicine, the Chinese Academy of Sciences, and the United Kingdom's Royal Society—to "discuss the scientific, ethical and governance issues associated with human gene-editing research."86
At the conclusion of the International Summit, the members of the organizing committee released a statement related to both basic research on and clinical use of gene editing (whether for therapy or research, and whether somatic or germline). The summit participants were supportive of basic research, including research using human embryos that would not be used to establish a pregnancy. They also supported the potential for the clinical use of human germline gene editing, with qualifications, stating that, "as scientific knowledge advances and societal views evolve, the clinical use of germline editing should be revisited on a regular basis."87
In early 2017, the NAS released a report titled Human Genome Editing: Science, Ethics, and Governance.88 The findings in this report largely align with those of the International Summit. The report does not propose an outright prohibition on germline genetic modification. Rather, it proposes a number of criteria that would have to be met for such research or clinical applications to move forward (e.g., after receiving societal consensus, only under strict oversight, and only for "compelling" reasons).89 In practical terms, these criteria have not been met yet. The position put forward both at the International Summit and in the 2017 NAS report on potential modification of the human germline represents a departure from earlier views on the subject, with this application of technology previously "viewed almost universally as a line that should not be crossed."90 More recently, a July 2018 Nuffield Council on Bioethics report, titled Genome Editing and Human Reproduction: Social and Ethical Issues, seems to generally agree with the NAS report, stating that "the potential use of genome editing to influence the characteristics of future generations could be ethically acceptable in some circumstances, but only if certain conditions are met."91 In November 2018, the Second International Summit on Human Genome Editing was held amidst the claim of the birth of the first genetically modified babies, and although that development was denounced, summit participants rejected a moratorium on germline gene editing research and instead recommended a "translational pathway to germline editing."92
Agricultural Development
While the CRISPR-Cas9 technology and other genome-editing tools have generated substantial international interest in their potential for biomedical research and clinical innovations, the versatile technology may also make significant contributions to global agriculture.93 CRISPR-Cas9 permits the introduction or deletion of genetic sequences with much greater precision than traditional plant and livestock breeding techniques or earlier methods of genetic engineering (GE).94 Plant biotechnologists see the CRISPR-Cas9 technology as offering the capacity to engineer changes in major food crops by substituting existing plant DNA sequences with desired ones, or by enhancing or suppressing particular gene expression.95 Conventional plant breeding for desired traits often involves cross-breeding with related wild species of the target plant. However, this approach also introduces genes that are not wanted. CRISPR-Cas9 allows the breeder to take only the gene of interest from the wild species and insert it at a precise location in the target organism to produce a new plant variety. In addition, this precision also reduces the plant breeding cycle by years through eliminating the time-consuming backcrossing procedure in conventional plant breeding and older GE techniques.
Through more precisely altering DNA, CRISPR-Cas9 and other genome engineering technologies have the potential to provide a level of control over plant genetic material that is unprecedented. Future crops created through these technological systems could include those with higher degrees of plant-pest control, plants with new and enhanced nutritional characteristics, and varieties that could be grown on marginal lands or in poor quality soils. Transgenesis—the introduction of foreign DNA into a plant genome—has characterized most commercial plant biotechnology innovation over the past 25 years. Most of the global acreage planted to GE crops today is in corn, cotton, soybean, and canola production. Pest resistance and/or herbicide tolerance traits are the dominant features engineered into these GE crops. While CRISPR-Cas9 permits similar transgenic manipulation, it does so with greater precision in the genome, and can involve more than a single gene insertion. New genetic variation can be created by identifying the precise DNA sequence modifications that are wanted in the cultivated variety, and then introducing them via the CRISPR-Cas9 system. By controlling the specific genetic variation introduced into the cultivated plant, CRISPR-Cas9 opens up a fundamentally new method of creating novel plant cultivars. For example, in 2014, Chinese researchers published a paper claiming the development of a strain of wheat that is resistant to powdery mildew, a fungal disease that affects a wide range of plants.96 CRISPR has also been used to modify the genes of a variety of other agricultural products, including rice, soybeans, potatoes, sorghum, oranges, and tomatoes.97
CRISPR-Cas9 is also being used to alter the genes of livestock. If successful, these efforts could yield substantial economic benefits. One application is focused on reducing the loss of livestock to disease by providing immunity to a virulent hemorrhagic virus that causes a deadly form of swine flu. A trial is underway in which a particular gene in domestic pigs is replaced by a gene present in warthogs that is believed to provide resistance to the virus.98 Other CRISPR-enabled livestock work includes more beefy and tender Brazilian cattle, chickens that produce only female chicks for egg-laying, cattle that reproduce only males for greater feed-to-meat efficiency, and hornless dairy cattle, an innovation that could result in economic benefits, increased safety for farm workers, and improvements in animal welfare.99
U.S. Regulation and Oversight of Agricultural Biotechnology
Under the Coordinated Framework for the Regulation of Biotechnology (see "The Coordinated Framework for the Regulation of Biotechnology," above), the three lead agencies involved in the regulation of agricultural biotechnology are the U.S. Department of Agriculture's Animal and Plant Health Inspection Service (APHIS), which regulates the importation, interstate movement, and field testing of GE plants and organisms that are or might be plant pests under the Plant Protection Act (PPA; 7 U.S.C. §7701 et seq.); the Food and Drug Administration, which regulates GE foods and GE animals mainly under the Federal Food, Drug and Cosmetic Act (FFDCA; 21 U.S.C. §301 et seq.); and the Environmental Protection Agency. The environmental safety of plants engineered to express a pesticidal protein fall under EPA's regulatory authority through the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA; 7 U.S.C. §136 et seq.).100
Animal and Plant Health Inspection Service
Over 30 applications for genetically modified plants, including those created through the CRISPR-Cas9 and other gene-editing systems, have been submitted to APHIS for approval since 2011. The regulatory question for APHIS is whether these plants are or could become plant pests, and thereby subject to regulation under the PPA. Genetically engineering a plant has largely been accomplished through the use of a soil bacterium—Agrobacterium tumefaciens—as the vector through which foreign DNA is introduced into the target plant. The genus Agrobacterium was long on the APHIS list of regulated items because of its natural ability to invade a plant and introduce its own DNA. That characteristic made it a very efficient way to genetically engineer a new plant variety. In practice, DNA sequences from A. tumefaciens were almost universally used in GE plant engineering. The use of A. tumefaciens in the transgenic process, and often the presence of A. tumefaciens DNA in the resulting plant, would generally be enough to subject the GE plant to regulation under the PPA.
Uncertainty in the regulatory process governing genome editing has been described as an impediment to innovation. On the one hand, plants created through the CRISPR-Cas9 system could be tightly regulated in similar fashion to the older GE technologies for trait development. Alternatively, CRISPR-Cas9 could be treated similarly to plants developed through traditional plant breeding and/or mutagenesis and remain unregulated articles. To address this uncertainty, Secretary of Agriculture Perdue issued a statement in March 2018101 that USDA has no plans to regulate plants that could otherwise have been developed through traditional breeding techniques as long as they are not plant pests or developed using plant pests. This would include plant varieties with the following changes:
- Deletions—modification of the plant is solely a genetic deletion of any size;
- Single base pair substitutions—modification of the plant is a single base pair substitution;
- Insertions from compatible plant relatives—modification of the plant is solely the introduction of a nucleic acid sequence from a compatible relative that could otherwise cross with the recipient organism and result in viable progeny through traditional plant breeding;
- Complete Null Segregants—progeny of genetically engineered plants that do not retain the change of the parent line.
For example, CRISPR-Cas9 was recently used to create a genetically modified mushroom that resists browning and a variety of specialty corn with unique starch characteristics ("waxy" corn).102 The two crops were created by using CRISPR-Cas9 "gene knock-out" technology to achieve the genetic transformation. Because the crops did not contain inserted genetic material from a donor organism, recipient organism, or vector agent meeting the definition of a plant pest, or was an unclassified organism or organism whose classification was unknown, APHIS asserted there was no basis to believe that the crops were or could become a plant pest within the meaning of the PPA. On this basis, APHIS determined in April 2016 that the agency had no regulatory authority under the PPA.103 The mushroom and waxy corn varieties thus became the first crops created by CRISPR-Cas9 to be approved by APHIS.104
Food and Drug Administration
Genomic editing for trait development in animals also introduces new regulatory uncertainties. FDA has regulatory authority over GE animals under its new animal drug protocol. To date, the agency has overseen the regulatory approval process of two species: a GE salmon and a GE mosquito.105 In 2017, the agency proposed guidelines for the genome-editing industry stating that each specific edit of an animal's genome would be treated as a new drug whose safety (and environmental impact) would have to be individually assessed.106 Researchers, particularly in smaller firms and academics, have asserted that such a regulatory approach could inhibit U.S. innovation in animal genomic research.107
In October 2018, FDA released the Plant and Animal Biotechnology Innovation Action Plan, which indicates the agency's intent to release a set of guidance documents over the next year that "will more clearly describe how the FDA is applying its regulatory oversight based on the risk profile of different types of products."108 According to the plan, the set of guidance documents will include (1) guidance for industry related to the "regulation of intentional genomic alterations in animals"; (2) guidance to clarify the agency's regulatory approach to gene edited animals used in research; and (3) guidance for industry "to establish an alternative type of file as a repository for information exchanges with the FDA's Center for Veterinary Medicine (CVM) for products that are in early development stages or that are developed for pure research and that may never progress to a marketable product."109 Additionally, FDA announced the establishment of a new pilot program, the Veterinary Innovation Program, which will provide "intensive assistance" to "increase the predictability of the regulatory pathway, facilitate a lower number of review cycles, and reduce the overall time to approval."110
Social Acceptance and Ethical Concerns
In some respects, current discussions of CRISPR-Cas9 are reminiscent of discussions over advances in genetic engineering in the 1980s. For example, at that time there were highly optimistic projections of being able to control photosynthesis, genetically engineer nitrogen fixing into plants, create "designer" foods with unique health properties, and cultivate plants on poor quality soils (e.g., aluminum toxicity). Potential social and environmental issues were noted in passing (e.g., weed and pest resistance, safety questions), but the technology's impressive promise and the fact that other countries were pushing ahead aggressively with development (e.g., Japan, Germany) left such issues largely in the background at the time. However, as products reached the market these issues resurfaced. Some issues remain unresolved as demonstrated by current debate over whether GE foods should be labeled for consumers. CRISPR-Cas9 is unlikely to escape similar social and ethical concerns as its use increases and evolves. For example, the use of CRISPR-Cas9 to create "gene-drive"—a method for spreading modified traits through wild populations over a few generations—has already sparked debate (discussed in more detail in "Gene Drives and Environmental Concerns," below).111 In 2014, a study by a group of biologists noted that gene drives based on CRISPR-Cas9 "could potentially prevent the spread of disease, support agriculture by reversing pesticide and herbicide resistance in insects and weeds, and control damaging invasive species."112 The study's authors noted that unwanted ecological effects would require careful assessment of each potential application. How such assessments would be done is an important policy issue.
Those opposed to "genetic engineering" regardless of the differences—or perhaps because of the differences—between CRISPR-Cas9 technology and conventional GE technologies are likely to continue raising issues surrounding human and environmental safety. The increased precision of genome engineering observed in the laboratory using CRISPR-Cas9 may have unknown effects when a CRISPR-modified plant is introduced into open environments with different agro-ecological characteristics. These concerns may need to be addressed systematically if the technology is to garner wider social acceptance. National and individual perceptions of risk vary and will continue to influence the development of CRISPR-Cas9 as they have with earlier technologies. Whether gene-edited plants will require specific labeling has not been decided. USDA's bioengineered food disclosure policy has yet to be finalized. However, the fact that USDA has stated it will not regulate genome edited plants suggests that foods containing such material may not be subject to disclosure.113
CRISPR-Cas9 and International Agriculture
The past 25 years of conventional GE agriculture may suggest how crop production based on CRISPR-Cas9 and gene editing could evolve in the coming years. The United States is the leading country in planting GE crops, accounting for more than 40% of acres growing GE crops worldwide. Elsewhere in the world the acceptance and cultivation of GE crops by both producers and consumers has been mixed. In the European Union (EU), for example, GE crops account for about 1% of crop acreage, all in a single variety of pest-resistant GE corn. This GE corn is cultivated mostly in Spain, with Portugal, the Czech Republic, Slovakia, and Romania having much smaller GE acreage. Several EU countries have completely banned the cultivation of GE crops in their territories or have specific rules on the trade of GE seeds. Only EU-approved varieties of GE commodities can be imported. All GE-derived food and feed must be labeled as such.
Public opinion in most EU member states remains strongly opposed to GE food and crops. Opposition in the EU may have influenced acceptance in other countries. Nine of the 14 developing countries that have approved commercial planting of GE crops are in Latin America. Most African countries have largely followed the EU in restricting or banning the cultivation of GE crops. South Africa, Egypt, Burkina Faso, and Sudan are the only African countries where GE crops are grown commercially. India, China, and Pakistan are major producers of GE cotton. The Philippines is the only Asian country to have approved a GE crop other than cotton for cultivation.
In contrast to the U.S. Secretary of Agriculture's recent statement that the United States will not regulate plants created through genomic editing (as long as they are developed without using a plant pest as the donor or vector, nor are plant pests themselves), the EU appears to be headed in the opposite direction, although in a direction similar to the one it has followed with transgenic organisms over nearly 20 years. On July 25, 2018, the Court of Justice of the European Union ruled that organisms obtained by mutagenesis are GMOs and are in principle within the scope of the obligations laid down in the GMO Directive, which governs the deliberate release into the environment of genetically modified organisms.114 While conventional mutagenesis techniques with a long safety record and used in a range of applications are not subject to the GMO Directive, the Court considers that risks posed by new mutagenesis techniques such as CRISPR-Cas9 might prove similar to risks from transgenesis. The Court considers that excluding organisms created from new mutagenesis techniques would compromise the objectives of the GMO Directive to avoid adverse effects on human health and the environment, and would also fail to respect the precautionary principal which the Directive seeks to implement.
In addition to variance in approval processes by different countries, trade negotiations concerning agricultural biotechnology also involve labeling issues for GE products and the difficulty of keeping GE material and non-GE material completely segregated in commodity supply chains. Harmonization of international trade regulations for products created through CRISPR-Cas9 could be as difficult to achieve as for conventional GE production.
Intellectual property issues surrounding CRISPR-Cas9 agricultural organisms are likely to continue to be a controversial issue in international agriculture. Given the dominance of a few agro-food corporations in seed development, questions related to who owns the raw material produced through gene editing and how the genome editing of global food crops is to be shared may be expected to continue. Agricultural productivity depends in part on the availability of biodiversity for the development of improved cultivars. Because genes can receive intellectual property protection, the emergence of CRISPR-Cas9 suggests that whole genomes could one day receive intellectual property protection as well. The objectives of the International Treaty on Plant Genetic Resources for Food and Agriculture (PGRFA), which was ratified by the U.S. Senate in September 2016, are the conservation and sustainable use of all plant genetic resources for food and agriculture, and the fair and equitable sharing of the benefits of their use.115 The purpose of the Multilateral System of the PGRFA is to facilitate access to plant genetic resources to ensure food security and fair distribution of the benefits from their use. CRISPR-Cas9 could add considerable complexity to implementing the PGRFA particularly in its stipulation of the right of contracting parties to save, use, exchange, and sell farm-saved seed.
Industrial Biotechnology
The potential impact of CRISPR-Cas9 on industries that rely on bacteria, fungi, and yeast is broad. First, CRISPR-Cas9 is broadening the number of microorganisms that could be used for industrial production.116 Second, CRISPR-Cas9 technology has been used to make industrially relevant strains resistant to viruses, to increase the production of chemicals used in biofuels, manufacturing, and to engineer probiotics.117 For example, researchers at the University of California, Riverside, have developed a yeast strain that can produce useful lipids and polymers, a development that some say may lead to the development of new precursors for biofuels, specialty polymers, adhesives and fragrances. This innovation is described as the first step to create long-chain hydrocarbons using yeast rather than synthetic processes. This approach offers the potential to replace non-renewable raw materials produced in petroleum refining processes with less expensive raw materials produced using a more efficient, safer bio-manufacturing process.118
Ecosystem Management and Conservation
CRISPR gene editing has been suggested as a potential control method to address the challenges posed by invasive species (e.g., spotted knapweed, Japanese beetles, and zebra mussels) and agricultural pests (e.g., Palmer amaranth).119 Specifically, the use of a gene drive has been proposed as a means to reduce populations of invasive or other unwanted species. As described above, a gene drive forces a trait that is present in a single individual to spread through an entire population in only a few generations.
A CRISPR-based gene drive could be used in various ways, including making an invasive species or an agricultural pest more susceptible to an herbicide or rodenticide, which would enable the species to be managed effectively by chemical control agents. It could also be used to bias the gender ratio of the invasive population towards males and therefore facilitate a decline in the population. For example, a sex-determining gene drive for invasive non-native species has been suggested as a method to preserve island biodiversity. Invasive species are the leading cause of extinction for native island species, and more than 80% of the world's islands have one or more invasive rodent species. Conventional control methods (i.e., trapping, the introduction of a predatory or parasitic species, and rodenticide application) are often labor intensive, cost-prohibitive, and indiscriminate (i.e., in many cases, native species can also be negatively affected by the control).120 A CRISPR-based gene drive is viewed by some as advantageous because it can be designed to be specific to the invasive species or targeted organism.121
Conversely, some researchers have announced plans to use CRISPR-Cas9 to recreate extinct species, including the wooly mammoth and the passenger pigeon. These de-extinction projects would compare the DNA of the extinct species to that of its modern relative and then edit the DNA of the contemporary animal to include the lost traits. For example, in the case of the wooly mammoth, the DNA of an Asian elephant would be altered to increase hair growth and subcutaneous fat.122
Gene Drives and Environmental Concerns
Anticipation of potential benefits of CRISPR-Cas9-enabled gene drives to human health, agriculture, and the environment is accompanied by concern over potential negative consequences to other species and ecosystems. According to a 2016 report by the National Academy of Sciences:
The fast moving nature of this field is both encouraging and concerning. While gene-drive modified organisms hold promise for addressing difficult to solve, persistent challenges, such as the eradication of vector-borne diseases and the conservation of threatened and endangered species, these proposed applications are based on limited proof-of-concept studies. The presumed efficiency of gene-drive modified organisms may lead to calls for their release in perceived crisis situations, before there is adequate knowledge of their ecological effects, and before mitigation plans for unintended harmful consequences are in place.123
Moreover, organisms that are invasive pests in one area (e.g., gray squirrels in Great Britain or mute swans in the United States) may be normal or even at risk in their native habitats (the eastern United States and western Europe, respectively). Transfer of organisms bearing the inserted genes from the target area to a non-target area could have unpredictable effects.
U.S. Regulation and Oversight of Gene Drives
Some experts have called for regulatory reform and clarity in how federal agencies will regulate the use of gene drives.124 The environmental release of gene-drive modified organisms will likely fall under the Coordinated Framework for the Regulation of Biotechnology (see previous section) with the responsible federal agency—the Environmental Protection Agency, the Food and Drug Administration, or the U.S. Department of Agriculture—identified based on the agencies' existing authorities and the intended use of the product (e.g., suppressing a target species or lowering disease transmission). Specifically, FDA regulates genetically engineered animals under the new drug provisions of the Federal Food, Drug and Cosmetic Act (FFDCA; 21 U.S.C. §301 et seq.); EPA regulates pesticides through the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA; 7 U.S.C. §136 et seq.); and the USDA Animal and Plant Health Inspection Service regulates genetically-engineered organisms that are noxious weeds or might be plant pests under the Plant Protection Act (PPA; 7 U.S.C. §7701 et seq.) However, according to the National Academy of Sciences and others, the Coordinated Framework does not clearly distinguish which agency should regulate the various applications of gene-drive modified organisms. Additionally, the National Academy indicates that some uses will likely result in jurisdictional overlap and recommends the development of an interagency process to quickly determine which agency should be the lead for a particular application area.125
In October 2017, FDA released guidance that clarifies when a genetically modified mosquito is considered a new animal drug and therefore regulated by FDA and when the modified mosquito is considered a pesticide and regulated by EPA. If the intended use of the genetically modified mosquito is to reduce the population of mosquitos (i.e., cause sterility or change the sex ratio of the population) then it is to be treated as a pesticide; however, if the use of the modified mosquito is to reduce the viral or pathogen load of the population of mosquitos—reducing disease transmission—it is to be treated as a new animal drug.126 According to the Brookings Institution, FDA's guidance should be expanded to cover not just mosquito populations, but all animal populations, as it is likely that CRISPR-enabled gene drives may be used in similar animal population management efforts in the future (i.e., to control the spread of invasive species or the transmission of disease through other insects or animals).127
Assessing Environmental Risk
Assessing environmental risk associated with the release of a gene-drive-modified organism into an open environment is determined by the federal agency tasked with the responsibility for regulating the organism. Specifically, FDA and USDA are required to examine environment risks under processes defined by the National Environmental Policy Act (NEPA, 42 U.S.C. 4321 et seq.), while EPA is required to conduct an ecological risk assessment process under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA; 7 U.S.C. §136 et seq.).
NEPA requires the preparation of an environmental impact statement (EIS) for any major federal action significantly affecting the quality of the human environment. An EIS provides a description of the proposed action and the existing environment, as well as analysis of the anticipated beneficial and adverse environmental effects of all reasonable alternatives.128
NEPA requires some level of analysis when environmental impacts are uncertain or thought not to be significant. Projects for which it is not initially clear whether impacts will be significant require the preparation of an environmental assessment (EA). An EA is a concise public document that analyzes the environmental impacts of a proposed federal action and provides sufficient evidence to determine the level of significance of the impacts.129 It is followed by either a Finding of No Significant Impact (FONSI) or a decision to prepare an EIS.
Judicial interpretation of NEPA ultimately determined that the act did not require agencies to elevate environmental concerns over other considerations. Rather, the courts determined, NEPA requires only that the agency take a "hard look" at a project's environmental consequences before taking action. If the adverse environmental effects of the proposed action are adequately identified and evaluated, the agency is not constrained by NEPA from deciding that other benefits outweigh the environmental costs.130
According to the National Academy of Sciences:
Some of the key strengths of [the] NEPA process are that it is a standard approach required by legislation, supports the collection of large amounts of information about a proposed activity, it has clear reporting requirements, and includes provisions for public input. The NEPA process is also widely recognized by the stakeholder community. The disadvantage of the NEPA process, however, is that it is a regulatory process and not a decision science approach. Neither an EA nor an EIS requires a clear formulation of the problem that provides a quantitative cause-effect model. Analyses conducted as part of the NEPA process are not required to be probabilistic or report quantitatively on uncertainty. These gaps would make it very difficult to [a] create testable hypothesis to conduct further research on gene-drive modified organisms and inform decision making.131
In contrast, according the National Academy of Sciences, ecological risk assessment allows for the quantification of probable outcomes and the ability to trace cause-effect pathways. Both of these, in addition to the ability of ecological risk assessment to identify sources of uncertainty, likely make it a more thorough tool for supporting public policy decisions about the use of gene drive technologies.132 However, some in industry may argue that the NEPA process is sufficient and that requiring ecological risk assessments has the potential to lengthen the approval process, leading to unnecessary delays and costs that could have a negative effect on public health.
Social Acceptance and Ethical Concerns
According to the National Academy of Sciences, "there is insufficient evidence available at this time to support the release of gene-drive modified organisms into the environment," and a considerable amount of research and evaluation is still necessary. These experts also indicate that any decision to release a gene-drive modified organism into the environment must be accompanied by a "reasonable level of assurance" that the potential risks have been adequately identified and studied and are outweighed by the potential benefits.133
For example, a gene drive could be constructed to suppress the population of an invasive plant species so that native plant species would be able to re-populate the ecosystem. However, the invasive plant may have assumed a critical role in the ecosystem, and its suppression may result in the sudden loss of habitat or a food source for native animals even if native species are eventually able to assume their previous ecological roles. If the native animal is an endangered or threatened species, then reducing its habitat (i.e., reducing the invasive plant species) could have negative consequences for the native animal and its recovery as required by the Endangered Species Act.134 Additionally, while the desired benefit of suppressing the invasive species is re-population by the native species, it could actually create an opportunity for an even more resilient invasive species to take its place.
In another scenario, a gene drive could be developed to modify a population of mosquitos so they can no longer host the Zika virus and thereby reduce the number of infants with serious defects at birth or emerging later in life. However, an unintended consequence of modifying the mosquito population could be that it then becomes a more susceptible host for a new or existing virus that may have an even greater negative impact on human health.
Some scientists have called for the development of reversal or immunization gene drives as a means to counter any unintended consequences with the open environment release of a gene drive-modified organism. These gene drives could be designed to revert the targeted organism back to its original genetic state or incorporate a genetic change into the organism that would prevent it from being susceptible or "immune" to the original gene drive.135
Besides the scientific questions of risk in making changes to complex and interwoven ecosystems, these examples raise a number of questions about the use of gene drives and what may be considered socially acceptable. Some may view the use of gene drives to benefit public health, especially in a time of crisis (i.e., an outbreak of a harmful virus), as appropriate. Others may view the possibility of eradicating a species as morally objectionable, regardless of the potential benefits to human health. Others may object to the use of gene drives entirely, and view any attempt to "control" nature as unwarranted. These views may also vary by community. For example, a society that is plagued by a serious disease may be more tolerant of the use of gene drives and the potential unintended consequences that may result than a community not affected by the disease being targeted. Variation in societal and ethical views suggest the need for public engagement and dialogue before any field testing or open environmental release of a gene-drive modified organism. Concerns about environmental justice and who will be responsible for addressing unanticipated public health or environmental harms may also be an issue as developing countries may be primary locations for the use of gene drives.
According to the National Academy of Sciences:
Engagement can facilitate mutual learning and shared decision making, support democracy and justice, help identify and assess potential benefits and harms, and provide a mechanism to explore difficult-to-articulate questions, such as the human relationship to nature…. The question is not whether to engage communities, stakeholders, and publics in decisions about gene drive technologies, but how best to do so. The outcomes of engagement may be as crucial as the scientific outcomes to decisions about whether to release of a gene-drive modified organism into the environment. Thus, engagement cannot be an afterthought; it requires effort, attention, resources, and advanced planning.136
International Regulation of Genetically Modified Organisms
The United Nations Convention on Biological Diversity (CBD), as implemented through the Cartagena and Nagoya Protocols, is the primary international agreement governing the development and use of genetically modified organisms. The CBD entered into force in 1993 and, at present, 168 nations have signed onto the treaty; the United States is not signatory to the CBD. The treaty states as major objectives the conservation of biological diversity and sustainable use of its components; fair and equitable sharing of the benefits arising out of the use of genetic resources; and appropriate transfer of relevant technologies. The Cartagena Biosafety Protocol, completed in 2000, applies to the transboundary movement, handling, and use of genetically modified organisms that may affect human health, the environment, or biological diversity. Under Article 17 of the protocol, a party to the agreement is required to take appropriate action to notify a potentially affected party "when it knows of an occurrence under its jurisdiction resulting in a release that leads, or may lead, to an unintentional transboundary movement of a living modified organism that is likely to have a significant adverse effect on the conservation and sustainable use of biological diversity."137
The Cartagena Protocol was developed mainly due to concerns related to genetically modified crops, but extension of the protocol and the CBD to synthetic biology, and similarly to gene drives, has recently been examined.138 In November 2018, at a meeting to update the CBD, government officials considered (but ultimately rejected) a resolution that would have prohibited the release of organisms modified using gene drives, including experimental releases.139 The proposed moratorium on gene drives caused some in the scientific community to issue a letter in opposition to the ban, stating that, "closing the door on research by creating arbitrary barriers, high uncertainty, and open-ended delays will significantly limit our ability to provide answers to the questions policy-makers, regulators, and the public are asking. The moratorium suggested at CBD on field releases would prevent the full evaluation of the potential uses of gene drives." On the other hand, some environmental groups supported the ban on gene drives, stating that, "gene drives threaten natural systems. If released experimentally into the environment they may spread engineered genes uncontrollably through wild and domesticated species. This could alter ecological systems and food webs, harm biodiversity and eradicate beneficial organisms such as pollinators."140
The CBD is predicated on the precautionary principle, which is generally understood to mean that if definitive scientific certainty is lacking, it is better to err on the side of caution. This approach is a source of concern for critics, who worry about the possible erection of trade restrictions that might be justified by the application of this concept. The United States is generally more tolerant than many other nations of scientific uncertainty and risk as they relate to innovation and emerging technologies; it does not operate its regulatory systems according to the precautionary principle.
The National Academy of Sciences indicates that since the United States is not a party to the CBD, it lacks a clear policy for engaging with other countries with different systems of governance in the release of gene-drive modified organisms. The NAS report also expresses concern that many of the countries where field testing and the environmental release of gene-drive organisms is likely to occur lack independent capacity to assess the safety of gene drive research, to undertake public engagement and societal dialogue, and to maintain regulatory institutions.141
Basic Research
CRISPR-Cas9 gene editing provides flexibility and new opportunities in basic research. For example, the modeling of disease in animals is an important tool in fundamental understanding of disease and the development of therapeutics. CRISPR-Cas9 has made the development of animal models of disease less labor intensive, more cost-effective, and more precise. Before CRISPR-Cas9, creating a new mouse disease model took approximately a year and cost tens of thousands of dollars, but with the CRISPR technology a new mouse model can be created within a month and at a fraction of the previous cost.142 CRISPR-Cas9 is also expanding the types of animals that can be used for basic research. For example, neurobiologists are using CRISPR-Cas9 to develop the tree shrew as a model for the human brain.143 Additionally, some countries, including China and Japan, are using the technology to position themselves as leaders in primate-related research, especially neuroscience.144 For example, scientists in China have used CRISPR-Cas9 to create monkey models of autism and cardiovascular disease.145
Beyond editing the genome (i.e., deleting and/or inserting genes), CRISPR-Cas9 is being used to regulate the expression of genes and the proteins they produce—providing additional insight into cellular systems and disease. The study of changes in gene expression without the modification of the underlying DNA is termed epigenetics. CRISPR-Cas9 offers researchers the first tool to precisely alter the epigenome, the chemical compounds attached to DNA. With this technique, researchers modify the CRISPR-Cas9 technology so that it does not cut the target gene, but instead attaches itself to the gene in a way that promotes or prevents gene expression. The modified technology can also be coupled with other components to create on-off switches and fluorescent molecules to allow visualization of gene expression in living organisms.146
National Security Concerns
In 2016, then-Director of National Intelligence James Clapper stated that advances in genetic engineering may raise significant national security concerns:
Research in genome editing conducted by countries with different regulatory or ethical standards than those of Western countries probably increases the risk of the creation of potentially harmful biological agents or products. Given the broad distribution, low cost, and accelerated pace of development of this dual-use technology, its deliberate or unintentional misuse might lead to far-reaching economic and national security implications.147
In 2017 and 2018, Director of National Intelligence Daniel Coats also highlighted national security concerns associated with genome editing and advances in biotechnology. In 2018, Director Coats stated: "New biotechnologies are leading to improvements in agriculture, health care, and manufacturing. However, some applications of biotechnologies may lead to unintentional negative health effects, biological accidents, or deliberate misuse."148
Just as CRISPR-Cas9 technology is lowering the cost and technological expertise required for biological research in general, the technology could do the same for biological weapons programs. In theory, advances in gene editing could be used to create novel pathogens or change the hardiness, resistance, infectivity, pathogenicity, or specificity of existing pathogens. However, current understanding of many of these traits and how they interact in particular pathogens may complicate making desired changes without also causing undesired changes. A 2016 conference concluded that "with regards to weapons relevance, the implications of gene editing technology are probably modest. But should a biological weapons program be started today, these technologies would likely become a part of it."149 Additionally, the concerns discussed above regarding potential inadvertent effects of ecological use of CRISPR-Cas9 linked gene-drive technology equally apply to the potential effects of its deliberate malign use.
In general, the United States addresses dual-use technologies by controlling proliferation through export controls and international agreements when possible and by mitigating the risks of proliferation through other activities such as deterrence, disruption, and preparedness. Given the current global availability of CRISPR-Cas9 technology and knowledge, export control regimes and international agreements designed to limit proliferation may be ill-suited for addressing national security concerns raised by gene editing.150 Current efforts aimed at mitigating the risks of biological weapons in general will also help mitigate the risks of biological weapons developed by gene editing. However, it may be possible to use gene editing to circumvent current mitigation strategies.
Demonstrating its dual-use nature, this technology is likely to play an important role in improving the development of medical countermeasures against both traditional and genetically engineered biological weapons. Thus, this technology may simultaneously address some national security concerns while raising others.
In July 2017, the Defense Advanced Research Projects Agency (DARPA) announced that the agency would invest $65 million over four years in a program called "Safe Genes" with the goal being to "gain a fundamental understanding of how gene editing technologies function; devise means to safely, responsibly, and predictably harness them for beneficial ends; and address potential health and security concerns related to their accidental or intentional misuse." According to DARPA, each of the funded research teams will pursue one or more of the following technical objectives:
- develop genetic constructs—biomolecular "instructions"—that provide spatial, temporal, and reversible control of genome editors in living systems;
- devise new drug-based countermeasures that provide prophylactic and treatment options to limit genome editing in organisms and protect genome integrity in populations of organisms; and
- create a capability to eliminate unwanted engineered genes from systems and restore them to genetic baseline states.151
Author Contact Information
Footnotes
| 1. |
For example, zinc finger nucleases (ZFNs) and transcription activator-like effector-based nucleases (TALENs). |
| 2. |
John Travis, "Making the Cut: CRISPR Genome-Editing Technology Shows Its Power," Science, vol. 350, no. 6267, December 2015, p. 1456. |
| 3. |
See, for example, Heidi Ledford, "CRISPR, the Disruptor," Nature, vol. 522, no. 7554, June 3, 2015, pp. 20-24. |
| 4. |
A genome is an organism's complete set of DNA, including all of its genes. |
| 5. |
Heidi Ledford, "CRISPR, the Disruptor," Nature, vol. 522, no. 7554, June 3, 2015, pp. 20-24. |
| 6. |
Other CRISPR systems refers to CRISPR gene editing technologies that use Cas-associated proteins other than Cas9. |
| 7. |
Prashant Mali, Kevin M. Esvelt, and George M. Church, "Cas9 as a Versatile Tool for Engineering Biology," Nature Methods, vol. 10, no. 10, October 2013, p. 962. |
| 8. |
For a more detailed description, see http://www.yourgenome.org/facts/what-is-genome-editing. |
| 9. |
DNA consists of four types of bases: adenine (A), thymine (T), guanine (G) and cytosine (C). The order, or sequence of these bases, in part, determines the phenotype or observable traits of an individual. |
| 10. |
For a more technical description of how CRISPR-Cas9 can be used to modify or alter the genome see https://www.addgene.org/crispr/guide/ |
| 11. |
Research and Markets, "CRISPR Market - Forecasts from 2018 to 2023," August 2018, https://www.researchandmarkets.com/research/zxx35w/global_crispr?w=5 |
| 12. |
Research and Markets, "Genome Editing Global Market-Forecast to 2022," September 2016, http://www.researchandmarkets.com/research/7r73wl/genome_editing. |
| 13. |
Markets and Markets, "Genome Editing/Genome Engineering Market Worth 6.28 Billion USD by 2022," November 2017, https://www.marketsandmarkets.com/PressReleases/genome-editing-engineering.asp. |
| 14. |
Zion Market Research, "Global CRISPR Genome Editing Market Will Grow USD 4,271.0 Million by 2024," August 2018, https://globenewswire.com/news-release/2018/09/04/1565088/0/en/Global-CRISPR-Genome-Editing-Market-Will-Grow-USD-4-271-0-Million-by-2024-Zion-Market-Research.html. |
| 15. |
Grand View Research, "Genome Editing Market Size to Reach $8.1 Billion by 2025," February 2017, http://www.grandviewresearch.com/press-release/global-genome-editing-market. |
| 16. |
Security and Exchange Commission, EDGAR database, Editas Medicine, 10-Q, filed November 8, 2018, https://www.sec.gov/Archives/edgar/data/1650664/000155837018008907/edit-20180930x10q.htm. |
| 17. |
Editas Medicine asserts that it owns "certain rights under 24 U.S. patents, 62 pending U.S. patent applications, four European patents and related validations, 35 pending European patent applications, 5 pending [Patent Cooperation Treaty] applications, and other related patent applications in jurisdictions outside of the United States and Europe." Editas Medicine, Securities and Exchange Commission 10-K filing for the year ending December 31, 2015, https://www.sec.gov/Archives/edgar/data/1650664/000155837016004455/edit-20151231x10k.htm. |
| 18. |
Google Finance, "Editas Medicine, Inc.," accessed on November 15, 2018, https://www.google.com/finance?q=NASDAQ:EDIT. |
| 19. |
Max Stendahl, "Latest Editas Research Pact Could Be Worth up to $1 Billion," Boston Business Journal, March 22, 2017, http://www.bizjournals.com/boston/news/2017/03/22/latest-editas-research-pact-could-be-worth-up-to.html; Allergan, plc, "Allergan and Editas Medicine Enter into Strategic R&D Alliance to Discover and Develop CRISPR Genome Editing Medicines for Eye Diseases," press release, March 14, 2017, https://www.allergan.com/news/news/thomson-reuters/allergan-and-editas-medicine-enter-into-strategic. |
| 20. |
Juno Therapeutics, Inc., "Juno Therapeutics and Editas Medicine Announce Exclusive Collaboration to Create Next-Generation CAR T and TCR Cell Therapies," press release, May 27, 2015, http://ir.editasmedicine.com/phoenix.zhtml?c=254265&p=irol-newsArticle&ID=2125229. |
| 21. |
Editas Medicine, Inc., "Editas Medicine Announces Scientific Multi-Year Collaboration with Fondazione Telethon and Ospedale San Raffaele," press release, June 28, 2016, http://ir.editasmedicine.com/phoenix.zhtml?c=254265&p=irol-newsArticle&ID=2189488. |
| 22. |
CRISPR Therapeutics AG, "CRISPR Therapeutics Raises Additional $38M as Part of Series B Financing," press release, June 24, 2016, http://crisprtx.com/news-events/news-events-press-releases-2016-06-24.php. |
| 23. |
Prepared remarks of Marijn Dekkers, Chairman of the Board of Management, Bayer AG, February 25, 2016, http://press.bayer.com/baynews/baynews.nsf/id/A7GCKX-Address-by-Dr-Marijn-Dekkers?Open&parent=Speeches&ccm=040, |
| 24. |
Google Finance, "Crispr Therapeutics AG," accessed on April 17, 2017, https://www.google.com/finance?q=NASDAQ:CRSP. |
| 25. |
Caribou Biosciences, Inc., http://cariboubio.com/about-us/origins; Caribou Biosciences, Inc., "Caribou Biosciences Raises $30 Million in Series B Funding," press release, May 16, 2016, http://cariboubio.com/in-the-news/press-releases/caribou-biosciences-raises-30-million-in-series-b-funding. |
| 26. |
Preetika Rana, Amy Dockser Marcus, and Wenxin Fan, "China, Unhampered by Rules, Races Ahead in Gene-Editing Trials," The Wall Street Journal, January 21, 2018, https://www.wsj.com/articles/china-unhampered-by-rules-races-ahead-in-gene-editing-trials-1516562360. |
| 27. |
The Broad Institute is a collaboration between the Massachusetts Institute of Technology and Harvard University focused on the use of genomics to advance human health. The Broad Institute has been awarded the first patent on CRISPR-Cas9 in the United States; however, the University of California has filed an appeal to overturn the decision. |
| 28. |
Monsanto Company, "Monsanto Announces Global Licensing Agreement with Broad Institute on Key Genome-Editing Application," press release, September 22, 2016, http://news.monsanto.com/press-release/corporate/monsanto-announces-global-licensing-agreement-broad-institute-key-genome-edi; Issi Rosen, Chief Business Officer, Broad Institute, "Licensing CRISPR for Agriculture: Policy Considerations," Broad Institute, https://www.broadinstitute.org/news/licensing-crispr-agriculture-policy-considerations. |
| 29. |
Calyxt, Inc., "University of Minnesota Grants Calyxt an Exclusive License," press release, July 28, 2016, http://www.calyxt.com/wp-content/uploads/2015/07/PR_Calyxt_UMN_7.28.15.pdf. |
| 30. |
For more information on OSTP, see CRS Report R43935, Office of Science and Technology Policy (OSTP): History and Overview, by [author name scrubbed] and [author name scrubbed]. |
| 31. |
Memorandum for Heads of Food and Drug Administration, Environmental Protection Agency, and Department of Agriculture, "Modernizing the Regulatory System for Biotechnology Products," Executive Office of the President, July 2, 2015. https://www.epa.gov/sites/production/files/2016-12/documents/modernizing_the_reg_system_for_biotech_products_memo_final.pdf. |
| 32. |
The discovery of CRISPR occurred at Japan's Osaka University in 1987, although the implications of the technology for genetic modification of organisms other than microbes were not recognized until researchers at Harvard, Vilnius University, University of California, Berkeley, and the Max Plank Institute in Germany developed a model in 2011-2012 that permitted genomic engineering of plants and animals. See Doudna, J.A. and Charpentier, E. "The New Frontier of Genome Engineering with CRISPR/Cas9," Science, vol. 346, issue 6213, November 28, 2014. DOI: 10.1126/science.1258096. |
| 33. |
Increasing the Transparency, Coordination, and Predictability of the Biotechnology Regulatory System, January 2017, https://obamawhitehouse.archives.gov/blog/2017/01/04/increasing-transparency-coordination-and-predictability-biotechnology-regulatory. |
| 34. |
The EU and most other countries that are signatories to the Cartagena Protocol—the international agreement governing the safe handling, transport and use of organisms derived from biotechnology—implemented biotechnology regulatory policies that are process-based. As the first country to approve a genetically modified crop, the United States adopted a product-based approach to regulation. |
| 35. | |
| 36. |
National Academies of Science, Engineering, and Medicine, Preparing for Future Products of Biotechnology, The National Academies Press. DOI: https://doi.org/10.17226/24605. |
| 37. |
Ibid. |
| 38. |
ResearchLABlog, Gene Editing to Treat Diabetes, May 25, 2016, http://researchlablog.org/post/144915391951/gene-editing-to-treat-diabetes. |
| 39. |
American Diabetes Association, The Cost of Diabetes, http://www.diabetes.org/advocacy/news-events/cost-of-diabetes.html. |
| 40. |
Andrew Hammond, Roberto Galizi, and Kyros Kyrou, "A CRISPR-Cas9 Gene Drive System Targeting Female Reproduction in the Malaria Mosquito Vector Anopheles gambiae," Nature Biotechnology, vol. 34 (2016), pp. 78-83. |
| 41. |
Jerry Adler, "Kill All the Mosquitoes?," Smithsonian Magazine, June 2016, http://www.smithsonianmag.com/innovation/kill-all-mosquitos-180959069/?no-ist. |
| 42. |
Heidi Ledford and Ewen Callaway, "'Gene Drive' Mosquitoes Engineered to Fight Malaria," Nature, November 23, 2015, http://www.nature.com/news/gene-drive-mosquitoes-engineered-to-fight-malaria-1.18858. |
| 43. |
Antonio Regalado, "Bill Gates Doubles His Bet on Wiping Out Mosquitoes with Gene Editing," MIT Technology Review, September 6, 2016, https://www.technologyreview.com/s/602304/bill-gates-doubles-his-bet-on-wiping-out-mosquitoes-with-gene-editing/; Target Malaria, "Our Work," https://targetmalaria.org/our-work/. |
| 44. |
Roll Back Malaria is a partnership initiated by the World Health Organization (WHO), the United Nations Development Program (UNDP), the United Nations Children's Fund (UNICEF), and the World Bank in 1998 to reduce the human and socioeconomic costs of malaria. |
| 45. |
Roll Back Malaria, "Economics of Malaria," http://www.rollbackmalaria.org/files/files/toolbox/RBM%20Economic%20Costs%20of%20Malaria.pdf. |
| 46. |
Centers for Disease Control and Prevention, "Impact of Malaria," https://www.cdc.gov/malaria/malaria_worldwide/impact.html. |
| 47. |
Hannah Osborne, "Malaria and Zika: CRISPR Gene Editing Could Wipe Out Blood-Sucking Female Mosquitoes," International Business Times, February 17, 2016, http://www.ibtimes.co.uk/malaria-zika-crispr-gene-editing-could-wipe-out-blood-sucking-female-mosquitos-1544426. |
| 48. |
ClinicalTrials.gov, "A Safety and Efficacy Study Evaluating CTX001 in Subjects with Severe Sickle Cell Disease," https://clinicaltrials.gov/ct2/show/NCT03745287?term=CRISPR&rank=6. |
| 49. |
Teresa L. Kauf, Thomas D. Coates, Liu Huazhi et al., "The Cost of Health Care for Children and Adults with Sickle Cell Disease," American Journal of Hematology, March 16, 2009, http://onlinelibrary.wiley.com/doi/10.1002/ajh.21408/epdf. |
| 50. |
Megan Molteni, "Crispr Halted Muscular Dystrophy in Dogs. Are Humans Next?," Wired, August 30, 2018, https://www.wired.com/story/crispr-halted-muscular-dystrophy-in-dogs-someday-it-might-cure-humans/. |
| 51. |
Duchenne Muscular Dystrophy is one of nine forms of muscular dystrophy. |
| 52. |
Margaret Wahl, "MDA Study Reveals 'Cost of Illness' for ALS, DMD, MMD," http://quest.mda.org/news/mda-study-reveals-cost-illness-als-dmd-mmd. |
| 53. |
Rodolphe Barrangou and Jennifer A. Doudna, "Applications of CRISPR Technologies in Research and Beyond," Nature Biotechnology, vol. 34, no. 9, September 2016, p. 937. |
| 54. |
Centers for Disease Control and Prevention, "Antibiotic Resistance Threats in the United States, 2013," https://www.cdc.gov/drugresistance/threat-report-2013/index.html. |
| 55. |
Rodolphe Barrangou and Jennifer A. Doudna, "Applications of CRISPR Technologies in Research and Beyond," Nature Biotechnology, vol. 34, no. 9, September 2016, p. 937. |
| 56. |
A germ line is the sex cells (eggs and sperm) that are used by sexually reproducing organisms to pass on genes from generation to generation. Egg and sperm cells are called germ cells, in contrast to the other cells of the body that are called somatic cells. National Human Genome Research Institute (NHGRI), https://www.genome.gov/glossary/index.cfm?id=94. |
| 57. |
ClinicalTrials.gov, "PD-1 Knockout Engineered T Cells for Metastatic Non-Small Lung Cancer," https://clinicaltrials.gov/ct2/show/NCT02793856?term=crispr&rank=4. |
| 58. |
David Cyranoski, "CRISPR Gene-Editing Tested in a Person for the First Time," Nature, vol. 539, iss. 7630, November 15, 2016. |
| 59. |
The purpose of a Phase 1 trial is to examine safety and dosage. The purpose of a Phase 2 trial is to examine efficacy and side effects. Some clinical trials are designed to combine two phases into a single trial, i.e. Phase 1/2. For more information on clinical research phases see https://www.fda.gov/ForPatients/Approvals/Drugs/ucm405622.htm. |
| 60. |
Sarah Reardon, "First CRISPR Clinical Trial Gets Green Light from US Panel," Nature News, June 22, 2016. |
| 61. |
ClinicalTrials.gov, "NY-ESO-1-redirected CRISPR (TCRendo and PD1) Edited T Cells (NYCE T Cells)," https://clinicaltrials.gov/ct2/show/NCT03399448?term=CRISPR&draw=3&rank=5. |
| 62. |
ClinicalTrials.gov, "A Safety and Efficacy Study Evaluating CTX001 in Subjects with Severe Sickle Cell Disease," https://clinicaltrials.gov/ct2/show/NCT03745287?term=CRISPR&rank=6. |
| 63. |
21 C.F.R. §312. |
| 64. |
Editas Medicine, "Editas Medicine Announces FDA Acceptance of IND Application for EDIT-101," November 30, 2018, http://ir.editasmedicine.com/news-releases/news-release-details/editas-medicine-announces-fda-acceptance-ind-application-edit. |
| 65. |
Puping Liang et al., "CRISPR-Cas9-Mediated Gene Editing in Human Tripronuclear Zygotes," Protein & Cell, vol. 6, issue 5, pp. 363-372, May 2015. |
| 66. |
Ewen Callaway, "Second Chinese Team Reports Gene Editing in Human Embryos," Nature News, April 8, 2016. |
| 67. |
H. Ma, N. Marti-Gutierrez, et al., "Correction of a Pathogenic Gene Mutation in Human Embryos," Nature, vol. 548, pps. 413-419, August 2017. |
| 68. |
See for example D. Egli, M.V. Zuccaro, et al, "Inter-Homologue Repair in Fertilized Human Eggs?," Nature, vol. 560, pps. E5-E7, 2018, https://www.nature.com/articles/s41586-018-0379-5. |
| 69. |
Lichun Tang et al., "CRISPR/Cas9-Mediated Gene Editing in Human Zygotes Using Cas9 Protein," Molecular Genetics and Genomics, pp. 1-9, http://link.springer.com/article/10.1007%2Fs00438-017-1299-z. |
| 70. |
Y. Zeng, J. Li, G. Li, et al., "Correction of the Marfan Syndrome Pathogenic FBN1 Mutation by Base Editing in Human Cells and Heterozygous Embryos," Molecular Therapy, vol. 26, issue 11, pps. P2631-P2637, November 7, 2018. |
| 71. |
Martin Jinek et al., "A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity," Science, vol. 337, issue 6096, pp. 816-821. |
| 72. |
For more information about NIH as well as federal funding for research and development, see CRS Report R43341, NIH Funding: FY1994-FY2019, by [author name scrubbed] and [author name scrubbed], and CRS Report R44516, Federal Research and Development Funding: FY2017, coordinated by [author name scrubbed] |
| 73. |
For more information about Labor-HHS-Education appropriations, see CRS Report R44691, Labor, Health and Human Services, and Education: FY2017 Appropriations, coordinated by [author name scrubbed] and [author name scrubbed]. |
| 74. |
NIH, "Statement on NIH Funding of Research Using Gene-Editing Technologies in Human Embryos," April 28, 2015, https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-funding-research-using-gene-editing-technologies-human-embryos. |
| 75. |
National Institutes of Health, "NIH Guidelines for Research Involving Recombinant of Synthetic Nucleic Acid Molecules (NIH Guidelines)," April 2016, http://osp.od.nih.gov/sites/default/files/resources/NIH_Guidelines.pdf. |
| 76. |
NIH Guidelines for Research Involving Recombinant of Synthetic Nucleic Acid Molecules (NIH Guidelines), Section I-A-1-a. IRBs review and monitor biomedical and behavioral research involving human subjects; IBCs provide local institutional oversight of recombinant DNA research. |
| 77. |
83 Federal Register 41082, August 17, 2018. |
| 78. |
F.S. Collins and S. Gottlieb, "The Next Phase of Human Gene-Therapy Oversight," New England Journal of Medicine, vol. 379, issue 15, pps. 1393-1395, October 11, 2018. |
| 79. |
National Institutes of Health, "Statement on Modernizing Human Gene Therapy Oversight," August 16, 2018, https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-modernizing-human-gene-therapy-oversight. |
| 80. |
Office of Science Policy, NIH, "Proposal to Streamline Review of Gene Therapy Trials and Restore the Original Vision of the RAC—August 2018," https://osp.od.nih.gov/biotechnology/nih-guidelines/. |
| 81. |
Section 508, P.L. 114-113. |
| 82. |
Section 734, P.L. 115-141. |
| 83. |
See for example FDA, "Chemistry, Manufacturing and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs)," July 2018, https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM610795.pdf. |
| 84. |
58 Federal Register 53248, October 14, 1993. |
| 85. |
Public Health Service Act, Section 351. |
| 86. |
The National Academies of Sciences, Engineering, and Medicine, "International Summit on Human Gene Editing," http://nationalacademies.org/gene-editing/Gene-Edit-Summit/. |
| 87. |
The National Academies of Sciences, "On Human Gene Editing: International Summit Statement," December 3, 2015, http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=12032015a. |
| 88. |
The National Academies of Sciences, Engineering, and Medicine, "Human Genome Editing: Science, Ethics, and Governance," 2017, https://www.nap.edu/catalog/24623/human-genome-editing-science-ethics-and-governance. |
| 89. |
The National Academies of Sciences, "With Stringent Oversight, Heritable Germline Editing Clinical Trials Could One Day Be Permitted for Serious Conditions; Non-Heritable Clinical Trials Should Be Limited to Treating or Preventing Disease or Disability at This Time," February 14, 2017, http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=24623. |
| 90. |
Nature Editorial, "Gene Politics: US Lawmakers Are Asserting Their Place in the Human Genetic-Modification Debate," Nature, vol. 523, issue 7558, July 1, 2015. |
| 91. |
Nuffield Council on Bioethics, "Genome Editing and Human Reproduction: Social and Ethical Issues," http://nuffieldbioethics.org/report/genome-editing-human-reproduction-social-ethical-issues/ethical-considerations-conclusions. |
| 92. |
The National Academies of Sciences, Engineering, and Medicine, "On Human Genome Editing II, Statement by the Organizing Committee of the Second International Summit on Human Genome Editing," November 29, 2018, http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=11282018b. |
| 93. |
CRISPR-Cas9 was first demonstrated in 2013 as a genome editing tool in Arabidopsis and tobacco. It was further tested in commercial crops such as wheat, rice, and soybeans, as well as several fruit and vegetable crops. In September 2016, Monsanto licensed the CRISPR-Cas9 technology from the Broad Institute, becoming the first licensee to do so for agricultural purposes. The DuPont Corporation also is developing drought tolerant corn and wheat varieties using CRISPR-Cas9 technology. |
| 94. |
Conventional genetic modification techniques are referred to as "genetic engineering" whereas the newer, "synthetic" biology, of which CRISPR-Cas9 is part, is referred to as "genome engineering." |
| 95. |
Qiwei Shan et al. "Targeted Genome Modification of Crop Plants Using a CRISPR/Cas9 System." Nature Biotechnology, vol. 31, pp. 686-688, August 8, 2013. DOI:10.1038/nbt.2650 |
| 96. |
Yanpeng Wang, Xi Cheng, Qiwei Shan et al., "Simultaneous Editing of Three Homoeoalleles in Hexaploid Bread Wheat Confers Heritable Resistance to Powdery Mildew," Nature Biotechnology, vol. 32, no. 9, July 20, 2014, pp. 947-951. |
| 97. |
Maywa Montenegro, "CRISPR Is Coming to Agriculture—With Big Implications for Food, Farmers, Consumers, and Nature," Ensia, January 28, 2016. |
| 98. |
University of Edinburgh, "Pigs Edited to Beat Virus Using Advanced Breeding Techniques," http://www.roslin.ed.ac.uk/news/2015/12/23/pigs-edited-to-beat-virus-using-advanced-breeding-techniques/ |
| 99. |
Ibid; Genetic Literacy Project, "Gene Edited Hornless Cow Improve Animal Welfare But Regulatory Fate Unclear," May 11, 2016, https://www.geneticliteracyproject.org/2016/05/11/gene-edited-hornless-cow-improve-animal-welfare-regulatory-fate-unclear; Daniel F Carlson, Cheryl A Lancto, Bin Zang et al., "Production of Hornless Dairy Cattle from Genome-Edited Cell Lines," Nature Biotechnology, vol. 34, no. 5, May 2016, pp. 479-481, http://www.nature.com/articles/nbt.3560. |
| 100. |
For more detail, see CRS Report RL32809, Agricultural Biotechnology: Background, Regulation, and Policy Issues, by [author name scrubbed]. |
| 101. |
U.S. Department of Agriculture, "Secretary Perdue Issues USDA Statement on Plant Breeding Innovation," press release, March 28, 2018, https://www.usda.gov/media/press-releases/2018/03/28/secretary-perdue-issues-usda-statement-plant-breeding-innovation. |
| 102. |
Waltz, E. "Gene-Edited CRISPR Mushroom Escapes U.S. Regulation." Nature, vol. 532, 292. April 14, 2016. DOI:10.1038/nature.2016.1975. Corn starch is composed of amylose and amylopectin. Waxy corn was created by inactivating the gene that produces amylose resulting in a corn that is exclusively composed of amylopectin. The resulting corn variety has superior physico-chemical properties for use in the food and paper industries. |
| 103. |
APHIS's regulations for genetically engineered organisms are codified at 7 C.F.R. 340 ("Introduction of Organisms and Products Altered or Produced Through Genetic Engineering Which Are Plant Pests or Which There Is Reason to Believe Are Plant Pests"). |
| 104. |
Technically, APHIS concluded that the two crops were not "regulated articles" subject to oversight under 7 C.F.R. 340. While APHIS concluded that it had no regulatory authority under the PPA to regulate the CRISPR-edited crops, there is a voluntary review of these crops under the FDA. |
| 105. |
CRS Report R43518, Genetically Engineered Salmon, by [author name scrubbed] and [author name scrubbed]; CRS In Focus IF10401, Genetically Engineered Mosquitoes: A Vector Control Technology for Reducing Zika Virus Transmission, by [author name scrubbed]. |
| 106. |
U.S. Food and Drug Administration. Draft Guidance for Industry: Regulation of Intentionally Altered Genomic DNA in Animals, January 2017, https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM113903.pdf. |
| 107. |
Kelly Servick, "Proposed U.S. Biotech Rules Raise Industry Hopes and Anxieties," Science News, January, 27, 2017, http://www.sciencemag.org/news/2017/01/proposed-us-biotech-rules-raise-industry-hopes-and-anxieties. |
| 108. |
U.S. Food and Drug Administration, Plant and Animal Biotechnology Innovation Action Plan, Silver Spring, MD, October 2018, p. 3, https://www.fda.gov/Safety/Biotechnology/ucm624416.htm. |
| 109. |
Ibid., p. 4. |
| 110. |
U.S. Food and Drug Administration, "VIP: Veterinary Innovation Program," https://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/BiotechnologyProductsatCVMAnimalsandAnimalFood/AnimalswithIntentionalGenomicAlterations/ucm620835.htm. |
| 111. |
Burt, A., "Site-Specific Selfish Genes as Tools for the Control and Genetic Engineering of Natural Populations," Proceedings of the Biological Sciences B, pp. 921-928, May 7, 2003. DOI: 10.1098/rspb.2002.2319. |
| 112. |
Kevin M. Esvelt. et al., "Emerging Technology: Concerning RNA-Guided Gene Drives for the Alternation of Wild Populations," eLife, July 17, 2014. DOI: http://dx.doi.org/10.7554/eLife.03401. |
| 113. |
The National Bioengineered Food Disclosure Standard (P.L. 114-216) has defined "bioengineered food" as food containing genetic material that has been modified through in vitro recombinant DNA techniques and "for which modification could not otherwise be obtained through conventional breeding or found in nature." The proposed rule on the Food Disclosure Standard makes no mention of whether ingredients from crops produced by genomic editing meet this definition of "bioengineered." |
| 114. |
Court of the European Union. Press Release No. 111/18, 25 July 2018. Judgment in Case C-528/16. https://curia.europa.eu/jcms/upload/docs/application/pdf/2018-07/cp180111en.pdf. |
| 115. |
See Treaty text at http://www.fao.org/3/a-i0510e.pdf. |
| 116. |
Paul D. Donohue, Rodolphe Barrangou, and Andrew P. May, "Advances in Industrial Biotechnology Using CRISPR-Cas Systems," Trends in Biotechnology, vol. 36, no. 2 (February 2018). |
| 117. |
Rodolphe Barrangou and Jennifer A. Doudna, "Applications of CRISPR Technologies in Research and Beyond," Nature Biotechnology, vol. 34, no. 9, September 2016, p. 938. |
| 118. |
Sarah Nightingale, "CRISPR-Cas9 Tool Expedites Production of Biofuel Precursors and Specialty Polymers in Living Systems," Phys.org, January 26, 2016, http://phys.org/news/2016-01-crispr-cas9-tool-production-biofuel-precursors.html. |
| 119. |
Kevin M. Esvelt et al., "Emerging Technology: Concerning RNA-Guided Gene Drives for the Alteration of Wild Populations," eLife, July 17, 2014, https://elifesciences.org/content/3/e03401. |
| 120. |
National Academies of Sciences, Engineering, and Medicine, Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values (Washington, DC: The National Academies Press, 2016). |
| 121. |
Nicholas Wade, "Gene Drives Offer New Hope Against Diseases and Crop Pests," The New York Times, December 21, 2015. |
| 122. |
Sara Reardon, "The CRISPR Zoo," Nature, vol. 531, March 10, 2016. |
| 123. |
National Academies of Sciences, Engineering, and Medicine, Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values (Washington, DC: The National Academies Press, 2016). |
| 124. |
Oye et al., "Regulating Gene Drives," Science, vol. 345, no. 6197, August 8, 2014, pp. 626-628. |
| 125. |
National Academies of Sciences, Engineering, and Medicine, Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values (Washington, DC: The National Academies Press, 2016), p. 8. |
| 126. |
Department of Health and Human Services, Food and Drug Administration, "Clarification of FDA and EPA Jurisdiction Over Mosquito-Related Products: Guidance for Industry," October 2017. |
| 127. |
Jack Karsten and Darrell M. West, "New Biotech Regulations Require Balance of Safety and Innovation," March 3, 2017, https://www.brookings.edu/blog/techtank/2017/03/03/new-biotech-regulations-require-balance-of-safety-and-innovation/. |
| 128. |
For more information, see CRS Report RL33152, The National Environmental Policy Act (NEPA): Background and Implementation, by [author name scrubbed]. |
| 129. |
40 C.F.R. §1508.9. |
| 130. |
Baltimore Gas & Electric Co. v. Natural Resources Defense Council, Inc., 462 U.S. 87, 97, 100 (1983) and Robertson v. Methow Valley Citizens Council, 490 U.S. 332 (1989). |
| 131. |
National Academies of Sciences, Engineering, and Medicine, Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values (Washington, DC: The National Academies Press, 2016), p. 108. |
| 132. |
Ibid., pp. 108-109. |
| 133. |
Ibid., pp. 4-9. |
| 134. |
Such a situation has already occurred, even in the absence of advanced gene technology. The endangered southwestern willow flycatcher adapted to the invasive tamarisk tree which has been displacing native willows, the preferred nesting site for the flycatcher. When a beetle feeding exclusively on tamarisk was introduced and began to proliferate, native willows could not rapidly repopulate, and the flycatcher lost important nesting habitat. For more information, see https://www.usgs.gov/news/new-study-details-endangered-southwestern-willow-flycatcher-habitat-and-new-threats. |
| 135. |
Kevin M. Esvelt et al., "Emerging Technology: Concerning RNA-Guided Gene Drives for the Alteration of Wild Populations," eLife, July 17, 2014. |
| 136. |
National Academies of Sciences, Engineering, and Medicine, Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values (Washington, DC: The National Academies Press, 2016), p. 141. |
| 137. |
Cartagena Protocol on Biosafety, "Unintentional Transboundary Movements and Emergency Measures," http://bch.cbd.int/protocol/cpb_art17.shtml. |
| 138. |
Molly Bond and Deborah Scott, "In an Engineered World, Who Benefits from Biological Diversity?" The Guardian, December 22, 2016, https://www.theguardian.com/science/political-science/2016/dec/22/in-an-engineered-world-who-benefits-from-biological-diversity. |
| 139. |
Ewen Callaway, "UN Treaty Agrees to Limit Gene Drives but Rejects a Moratorium," Nature, November 29, 2018, https://www.nature.com/articles/d41586-018-07600-w. |
| 140. |
Antonio Regalado, "United Nations Considers a Test Ban on Evolution-Warping Gene Drives," MIT Technology Review, November 13, 2018, https://www.technologyreview.com/s/612415/united-nations-considers-a-test-ban-on-evolution-warping-gene-drives/; https://genedrivenetwork.org/open-letter; http://www.etcgroup.org/sites/www.etcgroup.org/files/files/call_to_protect_food_systems_oct_17th.pdf. |
| 141. |
National Academies of Sciences, Engineering, and Medicine, Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values (Washington, DC: The National Academies Press), p. 161. |
| 142. |
Heidi Ledford, "Riding the CRISPR Wave," Nature, vol. 531, March 10, 2016, p. 159. |
| 143. |
Sara Reardon, "The CRISPR Zoo," Nature, vol. 531, March 10, 2016, p. 163. |
| 144. |
Heidi Ledford, "Riding the CRISPR Wave," Nature, vol. 531, March 10, 2016, p. 159. |
| 145. |
David Cyranoski, "Monkey Kingdom," Nature, vol. 532, April 21, 2016, pp. 300-302. |
| 146. |
Heidi Ledford, "Riding the CRISPR Wave," Nature, vol. 531, March 10, 2016, p. 159. |
| 147. |
James R. Clapper, Director of National Intelligence, "Statement for the Record: Worldwide Threat Assessment of the US Intelligence Community," U.S. Senate Committee Armed Services, February 9, 2016. |
| 148. |
Daniel R. Coats, Director of National Intelligence, "Statement for the Record: Worldwide Threat Assessment of the US Intelligence Community," U.S. Senate Committee Armed Services, March 6, 2018. |
| 149. |
Speiz Laboratory, Spiez Convergence: Report on the Second Workshop (Spiez Laboratory: Swiss Federal Office for Civil Protection, 2016), pp. 21-26. |
| 150. |
See CRS Report RL33865, Arms Control and Nonproliferation: A Catalog of Treaties and Agreements, by [author name scrubbed], [author name scrubbed], and [author name scrubbed]. |
| 151. |
Defense Advanced Research Projects Agency, "Building the Safe Genes Toolkit," press release, July 18, 2017, https://www.darpa.mil/news-events/2017-07-19. |