Introduction
Individual nation states have developed intellectual property rights (IPR) regimes reflecting their domestic needs and priorities, although the United States and other countries have acceded to several IP-related conventions and treaties since the 1800s. Over time, IPR protection and enforcement have come to the forefront as a key international trade issue for the United States—largely due to the role of intellectual property in an innovative U.S. economy and as a U.S. competitive advantage—and figure prominently in the multilateral trade policy arena and in regional and bilateral U.S. free trade agreements (FTAs).
Congress has legislative, oversight, and appropriations responsibilities related to IPR and trade policy more generally. This role of Congress stems from the U.S. Constitution, which provides Congress with the power to "promote the Progress of Science and useful Arts, by securing for limited Times to Authors and Inventors the exclusive Right to their respective Writings and Discoveries" and to "regulate Commerce with foreign Nations."1 Since 1988, Congress has included IPR as a principal U.S. trade negotiating objective, and has passed laws such as "Special 301" to advance protection and enforcement of U.S. IPR in global markets. The context for congressional interest may include policy concerns such as: the role of IPR in the U.S. economy; the impact of IPR infringement on U.S. commercial, health, safety, and security interests; the effect of foreign indigenous innovation and localization requirement on U.S. IPR; and the balance or relationship between protecting IPR to stimulate innovation and advancing other public policy goals.
This report discusses the different types of IPR and IPR infringement, the role of IPR in the U.S. economy, estimated losses associated with IPR infringement, the organizational structure of IPR protection, U.S. trade policy, and issues for Congress regarding IPR and international trade.
IPR Definitions
Types of IPR
IPR are legal rights granted by governments to encourage innovation and creative output. They ensure that creators reap the benefits of their inventions or works. They take a variety of forms, such as patents, trade secrets, copyrights, trademarks, or geographical indications. Through IPR, governments grant a temporary legal monopoly to innovators by giving them the right to limit or control the use of their creations by others. IPR may be traded or licensed to others, usually in return for fees and/or royalty payments. Although the World Trade Organization (WTO) Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement) provides minimum standards for IPR protections, such rights are granted on a national basis and are, in general, enforceable only in the country in which they are granted. However, WTO members are obligated to abide by WTO rules, and their IPR enforcement practices can be challenged by other WTO members through the WTO dispute settlement process.
Patents
The Patent Act (Title 35 of the United States Code) governs the issuance and use of patents in the United States. Patents are granted for inventions of new products and processes (known as utility patents). Patents also may be granted for new designs and plant varieties. For an invention to be patentable, it must be new and "non-obvious" (involving an inventive step), and have a potential industrial or commercial application. The patent provides the holder with the exclusive right to exclude others from making, using, selling, or importing into the United States the patented invention for a period of 20 years.2 The patent right is based on the proposition that granting inventors a temporary monopoly over their invention will encourage innovation and promote the expenditure of money on research and development (R&D). The temporary monopoly may allow a patent holder to recoup these up-front costs by charging higher prices for the patented invention. In return for this economic rent, the patent holder must disclose the content of the invention to the public, along with test data and other information concerning the invention. This is meant to spur further creativity by those seeking to build on the patent after its expiration. Domestically, patents are granted by the U.S. Patent and Trademark Office (PTO) of the Department of Commerce.
Trade Secrets
Any type of valuable information, including a "formula, pattern, compilation, program, device, method, technique, or process," may be kept by its owner as a trade secret. To be a trade secret, the information must derive independent economic value from not being generally known or readily ascertainable by others, and be subject to reasonable efforts by the owner to maintain its secrecy.3 Examples of trade secrets include blueprints, customer lists, pricing information, and source code. While protection of patents and copyright is an exclusive matter of federal law, trade secret protection is found not only in federal law, but also in state law. Most states have adopted the Uniform Trade Secret Act (UTSA), a model law drafted by the National Conference of Commissioners on Uniform State Laws.
There are important differences between trade secrets and patents. Individuals do not have to apply for trade secret protection as they would for patents. Protection of trade secrets originates immediately with the creation of the trade secret; there is no process for applying for protection or registering trade secrets. Trade secret protection does not expire unless the trade secret becomes generally known. In contrast, patent applicants must disclose information about their innovation to the PTO in order to acquire a patent. The scope of protection is also different: patents preclude almost all uses of the invention by others, whereas trade secret law only prevents acquisition or misappropriation of a trade secret by improper means, such as theft. Patents thus offer right holders stronger protection but for a limited period of time. While applying for a patent can be a costly and lengthy process, patents are valuable if the confidentiality of the innovation is fragile (e.g., if the invention is easily reversed engineered) or if the area of research is highly competitive.
Copyright
Protection of copyrights in the United States is based on the Copyright Act (Title 17 of the United States Code). Copyrights protect original expressions of authorship, fixed in physical and/or digital forms. Such protections include literary or artistic works such as books, music, sound recordings, movies, paintings, architectural works, and computer code, and (in some cases) databases. Traditionally, copyrights differed from patents in that there was no claim to industrial applicability or novelty of the idea. The expression of the idea—the particular way it was conveyed in words, images, or sounds—and not the idea itself, was being copyrighted. While some of the criteria for copyrights differ from those of patents, the objective is the same: furthering creativity by promoting investments of time, money, and effort to create works of cultural, social and economic significance. U.S. law provides copyright protection for life of the author plus 70 years for personal works, or 120 years from creation (or 95 years from publication) for corporate works. Copyrights may be registered by the U.S. Copyright Office of the Library of Congress, although protection arises immediately upon fixation in a tangible medium of expression.
Trademarks
Trademark protection in the United States is governed jointly by state and federal law. The main federal statute is the Lanham Act of 1946 (Title 15 of the United States Code). Trademarks permit the seller to use a distinctive word, name, symbol, or device to identify and market a product or company. Marks can also be used to denote services from a particularly company. The trademark allows quick identification of the source of a product, and for good or ill, can become an indicator of a product's quality. If for good, the trademark can be valuable by conveying an instant assurance of quality to consumers. Trademark law serves to prevent other companies with similar merchandise from free-riding on the association of quality with the trademarked item. Thus, a trademarked good may command a premium in the marketplace because of its reputation. To be eligible for a trademark, the words or symbol used by the business must be sufficiently distinctive; generic names of commodities, for example, cannot be trademarked. Trademark rights are acquired through use or through registration with the PTO.
A related concept to trademarks is geographical indications (GIs), which are also protected by the Lanham Act. The GI acts to protect the quality and reputation of a distinctive product originating in a certain region; however, the benefit does not accrue to a sole producer, but rather the producers of a product originating from a particular region. GIs are generally sought for agricultural products, or wines and spirits. Protection for GIs is acquired in the United States by registration with the PTO, through a process similar to trademark registration.
Theft of Intellectual Property
Infringement
IPR infringement is the misappropriation or violation of the IPR. In the case of patents, infringement of a patent owner's exclusive rights involves a third party's unauthorized use, sale, or importation of the patented invention. Copyright infringement occurs when a third party engages in reproducing, performing, or distributing a copyrighted work without the consent of the copyright owner. The greatest challenge to the patent right in the context of international trade is infringement in foreign countries, or non-observance by WTO member states of the minimum standards of the TRIPS Agreement. In addition to the term infringement, other terms are used to describe certain violations of IPR.
Piracy
The term "piracy" generally refers to copyrights and generally refers to widespread, intentional infringement. The major challenge facing copyright protection is piracy, either through physical duplication of the work, illegal dissemination of copyrighted material (such as computer software, music, or movies) over the internet, and/or participation in commercial transactions of copyrighted materials without the consent of the copyright owner. Piracy can also mean the registration or use of a famous foreign trademark that is not registered in the country or is invalid because the trademark has not been used.
Counterfeiting
An imitation of a product is referred to as a "counterfeit" or a "fake." Counterfeit products are manufactured, marketed, and distributed with the appearance of being the genuine good and originating from the genuine manufacturer.4 The purpose of counterfeit goods is to deceive consumers about their origin and nature, harming both the trademark owner and consumers. Counterfeiting and copying of original goods are major challenges for trademarked products. The counterfeited product can be sold for a premium because of its association with the original item, while reducing the sales of the original items. Consumer experience with a counterfeited good of inferior quality can damage the reputation of the trademark product. Additionally, counterfeited goods of inferior quality may be potentially harmful to health and safety. Popular examples of counterfeit products include fake fashionwear (e.g., counterfeits of brand-name bags and watches) or fake pharmaceutical products (e.g., counterfeits of brand-name prescription medicines).
Trade Secret Theft
Misappropriation of trade secrets is a civil violation under federal and state laws. Theft of trade secrets may also be a federal crime in some circumstances. Industrial espionage refers to the stealing of trade secret information that relates to a product in interstate or foreign commerce, to the economic benefit of third parties and to the injury of the trade secret owner (18 U.S.C. 1832). Economic espionage refers to the stealing of a trade secret when the intent to benefit a foreign power (18 U.S.C. 1831).5 Trade secret theft can occur through cyber means (see below).6
Cybertheft
Criminal activity, including IP theft, increasingly occurs in the online environment. Internet-related crimes are often referred to as cybercrime, though no one definition appears to exist for it within the U.S. government.7 One of type of cybercrime is cybertheft, which broadly may be defined as crimes in which a computer is used to steal money or other things of value and can include "embezzlement, fraud, theft of intellectual property, and theft of personal and financial data."8 Other terms that may encompass internet-related IPR theft include cyber intrusions and cyberattacks.
Innovation Indicators
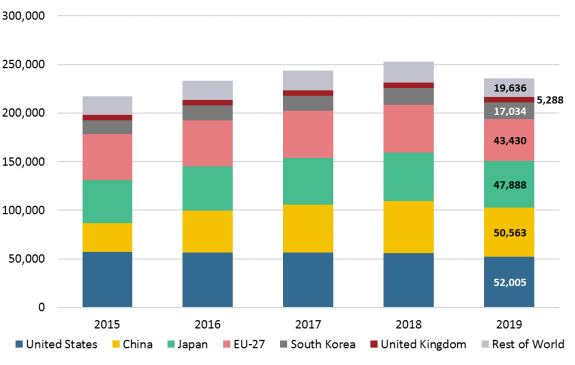
According to the Organization for Economic Co-operation and Development (OECD), innovation is the "implementation of a new or significantly improved product (good or service), or process, a new marketing method, or a new organizational method." Possible innovation-related indicators include activities concerning commercializing inventions and new technologies.9 Trends in the total number of patent applications under the Patent Cooperation Treaty (PCT), an international patent filing system administered by the World Intellectual Property Organization (WIPO), may be illustrative (see Figure 1).10 The United States remains the source of the world's largest number of PCT filing applications, followed by China and Japan; together, these three countries accounted for almost 64% of all PCT applications filed in 2019. China overtook the European Union (EU) and Japan in 2017.11 While China has become a top patent filer, the number of patents (quantity) does not necessarily reflect leadership in patent quality and innovativeness.12 The top fields of technology in PCT filings were digital communication, computer technology, audio-visual technology, electrical machinery/apparatus/energy, and optics.13
Role of IP in U.S. Economy and Trade
Intellectual property generally is viewed as a long-standing strategic driver of U.S. productivity, economic growth, employment, higher wages, and exports. It also is considered a key source of U.S. comparative advantage, such as in innovation and high-technology products. Nearly every industry depends on it for its businesses. Industries that rely on patent protection include the aerospace, automotive, computer, consumer electronics, pharmaceutical, and semiconductor industries. Copyright-reliant industries include the software, data processing, motion picture, publishing, and recording industries. Trademarks and trade secrets are widely used in most industries, but certain industries are especially trademark-intensive, including the apparel, pharmaceuticals, and electronics industries.14 Other industries that directly or indirectly benefit from IPR protection include retailers, traders, and transportation businesses, which support the distribution of goods and services derived from intellectual property.15
Overall Role
IP-intensive industries play a major role in the U.S. economy and international trade. What follows are some findings from a 2016 study by the U.S. Department of Commerce.16
- U.S. economic impact. In 2014, a subset of the most intellectual property-intensive industries directly supported 27.9 million jobs in the United States, or about 18% of total U.S. employment. They also indirectly supported 17.6 million U.S. jobs via the supply chain in other industries. In 2014, the wages of employees working in IP-intensive industries tended to be about 46% higher on average than those working in non-IP-intensive industries. These industries accounted for about $6.6 trillion in value added to the U.S. economy, more than one-third of the U.S. gross domestic product (GDP).
- U.S. trade in goods. In 2014, IP-related merchandise exports amounted to $842 billion (52% of total U.S. merchandise exports), while IP-related merchandise imports reached $1,391 billion (about 70% of total U.S. merchandise imports). Key sectors for IP-intensive merchandise exports include semiconductor and electric parts, basic chemicals, pharmaceuticals and medicine, measuring and medical instrument, and computer and peripheral equipment.17
- U.S. trade in services. In 2012, exports of services by IP-intensive industries totaled about $81 billion (about 12% of total U.S. private services exports). Key sources of services exports included the software publishing, financial services, computer systems design and related services, motion picture and video, and management and technical consulting industries. The study did not provide information on imports of services by IP-intensive industries, though it should be noted that the United States runs an overall surplus in international trade in services.18
Royalty and Licensing Charges
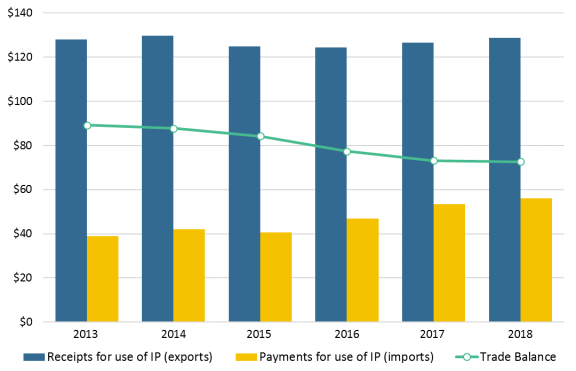
The role of IP-intensive industries in U.S. trade in services includes charges for U.S. IP, i.e., receipts (exports) and payments (imports) of royalties and licensing fees. Rights holders may authorize the use of technologies, trademarks, and entertainment products that they own to entities in foreign countries, resulting in revenues through royalties and license fees. Between 2013 and 2018, U.S. receipts for use of royalties and licensing fees have remained relatively steady while there has been a slight increase in payments from U.S. firms to foreign firms. In 2018, U.S. receipts from cross-border trade in royalties and license fees (relating to patent, trademark, copyright, and other intangible rights) totaled $129 billion, while U.S. payments of royalties and license fees to foreign countries amounted to $56 billion, resulting in a trade surplus of $73 billion (see Figure 2).
|
Figure 2. U.S. Trade in Services: Royalties and License Fees from Intellectual Property Use, 2013-2018 (billions of U.S. dollars) |
 |
|
Source: BEA, U.S. International Services data. |
Specific U.S. Industries
Industry-specific figures may further demonstrate the role of IP in the U.S. economy. For example:
- Copyright industries. According to a study commissioned by the International Intellectual Property Alliance (IIPA), in 2017, industries categorized as part of the "core" copyright industries (e.g., computer software, videogames, books, newspapers, periodicals and journals, motion pictures, recorded music, and radio and television broadcasting) contributed about $1.3 trillion to the U.S. economy ("value-added" to current GDP), representing about 6.9% of the U.S. economy. The study also estimated that the "core" copyright industries employed nearly 5.7 million workers in 2017, representing about 4% of the total U.S. workforce. In addition, the study estimated that foreign sales of certain U.S. copyright sectors totaled $191.2 billion in 2017.19
- Pharmaceutical industry. Between 1998 and 2019, employment in the industry grew 26%. According to the Pharmaceutical Researchers and Manufacturers of America (PhRMA), in 2017, American biopharmaceutical companies supported more than 800,000 jobs in R&D and more than 4 million jobs in total, when accounting for indirect jobs (vendors and suppliers) and induced jobs (additional private economic activity).20 According to PhRMA, R&D investment was about $97 billion in 2017.21
- Manufacturing industry: Based on data from a study by NDP Analytics, a private-sector research firm, IP-intensive manufacturing industries performed better than non-IP-intensive industries when comparing key economic measures: R&D investment, wages, exports, value-added, and gross output.22 For example, in 2015, the study estimated that exports per employee for IP-intensive manufacturing industries averaged about $177,033, compared to about $63,778 on average for non-IP-intensive manufacturing industries.23
- Software industry: Software.org, an independent research organization, reported that the software industry directly employs around 3 million workers and more than 14 million when accounting for indirect jobs in 2018. The report also stated that the industry directly contributed $845 billion in value-added to the U.S. GDP and invested almost $83 billion in R&D.24
"Fair Use" Industries
Some advocacy groups assert that empirical analysis of the role of IPR in the U.S. economy may not fully evaluate the economic and commercial benefits of lawful exceptions and limitations to exclusive rights—referred to broadly as "fair use." The "fair use" doctrine provides limitations and exceptions to the exclusive rights afforded by copyright law. It permits limited use of copyrighted works without requiring permission from the right holder in certain cases, examples of which may include news reporting, research, teaching, and library use.25 For example, by one estimate, in 2014, businesses that rely on "fair use" exceptions to U.S. copyright law generated total revenue of $5.6 trillion on average and $2.8 trillion on average of value-added (16% of total U.S. current dollar GDP).26 Additionally, employment associated with "fair use" totaled around 18 million of U.S. employment in 2014, and U.S. exports associated with "fair use" totaled $368 billion in 2014.27
Quantifying IPR Infringement
Advances in information and communications technology (ICT) and declining costs of transportation, spurred by lower trade barriers, have fundamentally changed information and trade flows. Such changes have created new markets for U.S. exporters, but at the same time, have been associated with the proliferation of counterfeiting and piracy on a global scale.
Several factors contribute to the growing problem of IPR infringement. While the costs and time for research and development are high, most IPR infringement occurs with relatively low costs and risks, and a high profit margin. According to PhRMA, it takes a pharmaceutical company over 10 years of R&D on average to create a new drug, with the average cost to develop a drug about $2.6 billion during the 2000s to early 2010s. In 2017, the biopharmaceutical industry invested around $91 billion for research and development in the United States.28 In contrast, drug counterfeiters can lower production costs by using inexpensive, and perhaps dangerous or ineffective, ingredient substitutes.
The development of technologies and products that can be easily duplicated, such as recorded or digital media, also has led to an increase in counterfeiting and piracy. Increasing internet usage has contributed to the distribution of counterfeit and pirated products. Additionally, civil and criminal penalties often are not sufficient deterrents for piracy and counterfeiting. The United States is especially concerned with foreign IPR infringement of U.S. intellectual property. Compared to foreign countries, IPR infringements levels in the United States are considered to be relatively low.29
Limitations on Data Estimating IPR Infringement Costs
Quantification of the economic losses associated with IPR infringement has been a long-standing focus in the academic, policy, and industry literature. Many experts agree that it is difficult to quantify the magnitude of IPR theft with any precision. Reasons may include
- Illicit nature of IPR infringement. Because IPR infringement is illicit and secretive, tools that are used to measure legitimate business activity cannot necessarily be used to measure economic losses from IPR infringement. As such, it may be easier to quantify the positive contribution of copyright industries to the U.S. economy more precisely than to measure the losses to the U.S. economy from copyright piracy.
- Quantifying specific components of economic impact. The economic impact of IPR infringement depends on a range of factors, including the different types of infringing goods being sold, the rate at which consumers substitute buying infringing goods for legitimate goods, and IPR infringement's deterrent effect on R&D and other investment. It may be difficult to measure precisely these components of the economic impact of IPR infringement.30
- Assumptions used to calculate economic impact. Methods for calculating data on counterfeiting and piracy often involve certain assumptions. Estimates of losses from IPR infringement can be highly sensitive to how these assumptions are derived and weighted. The basic economic model employed in some IPR loss estimates assumes that there is substitutability between pirated and legitimate goods. For example, under this model, sales of pirated goods may be equated to revenue losses of legitimate U.S. copyright businesses. Some analysts suggest that legitimate firms face a competition threat only if the individuals purchasing IPR-infringing products would be able and willing to purchase the legitimate product at the price offered when IPR infringement is not present.31 For consumers in developing countries, especially, this assumption may not be tenable.
- IPR infringement in the digital environment. While IPR infringement in the past primarily constituted counterfeiting and piracy of physical goods (such as CDs and books), there has been a growing amount of piracy taking place through digital mediums (such as illegal downloading and streaming of music, movies, and books over the internet). The use of virtual private networks (VPN) also makes it harder to track down the original location of infringement. It may be more complex to measure IPR infringement that takes place in the digital environment, and in turn, more difficult to measure the associated economic losses accurately. Quantifying the economic cost of trade-secret theft may be hampered by the reluctance of companies to disclose such theft, as well as difficulties assessing the monetary value of the secrets stolen. U.S. trade losses due to copyright infringement may be higher than reported because estimates often do not account for all forms of piracy, such as internet piracy. One study estimates that nearly 24% of global internet traffic infringes on copyright.32
- Sources of data. Estimates on economic losses from IPR infringement come from a range of sources, including academic, policy, and industry sources. According to a U.S. Government Accountability Office (GAO) study, the U.S. government does not systematically collect data or analyze the impacts of counterfeiting and piracy on the U.S. economy. In many cases, the federal government relies on estimates conducted by industry groups. However, companies may be reluctant to disclose their IPR losses because of possible reputational and commercial risks, and industry associations may not always release their proprietary data sources and methods, complicating efforts to verify such estimates.33
International Economic Effects
While assessments of the overall global economic costs of infringement on copyrights, trademarks, and patents are limited, available evidence indicates that the adverse economic effects of global IPR infringement stand in the hundreds of billions of dollars, and are increasing. Customs data on seizures of counterfeit and pirated goods may offer some idea of the magnitudes involved in terms of impact on producers and exporters.
A 2019 study jointly conducted by the Organization for Economic Cooperation and Development and the EU Intellectual Property Office (EUIPO) examined international trade in counterfeit goods using customs seizure data for 2014 through 2016.34 The OECD/EUIPO study estimated that the value of international trade in counterfeit and pirated goods was as much as $509 billion (equivalent to 3.3% of world trade) in 2016, up from the estimated $461 billion (2.5% of world trade) in 2013, according to a 2016 joint OECD-EUIPO study.35 The study also noted the industries impacted by IP infringement increased when compared to a previous study: products seized by customs between 2014 and 2016 covered 92% of Harmonized System (HS) chapters compared to 80% for the 2011 to 2013 period.36 OECD noted the significant increase in the use of small parcels as the form of delivery, which presents more challenges for customs officials to detect counterfeit and pirated goods. According to a 2017 OECD study that estimated trade of counterfeit and pirated information and community technology (ICT) goods, fake ICT goods accounted for up to 6.5% of total ICT trade and almost 43% of seized goods infringed the IP rights of U.S. firms.37 Counterfeit ICT goods may be consumer electronics, communication equipment, and electronic components.
Building on the 2016 OECD-EUIPO work is a study commissioned by the Business Action to Stop Counterfeiting and Piracy (BASCAP), a business initiative organized by the International Chamber of Commerce. According to BASCAP, the total value of counterfeit and pirated products was an estimated $923 billion to $1.1 trillion in 2013, and is projected to reach $1.9 to $2.8 trillion in 2022 (see Table 1).38
Table 1. Estimated International Economic Losses Due to Counterfeiting and Piracy, Selected Years
(billions of U.S. dollars)
|
Category |
2013 |
2022 |
|
Internationally traded counterfeit and pirated products |
$461 |
$991 |
|
Domestically produced and consumed counterfeit and pirated products |
$249-456 |
$524-959 |
|
Digitally pirated products |
$213 |
$384-856 |
|
Total |
$923-1,130 |
$1,900-2,810 |
Source: Frontier Economics, The Economic Impacts of Counterfeiting and Piracy, A Report Commissioned by Business Action to Stop Counterfeiting and Piracy (BASCAP), February 2017.
Notes: BASCAP economic loss estimates are restricted to the 35 OECD member countries.
U.S. Economic Effects
While specific estimates vary, the available data suggest that U.S. economic losses from IPR infringement are significant.
Customs Seizure Data
Data on pirated and counterfeit seizures of imports at U.S. borders by the Department of Homeland Security (DHS) shed light on the magnitude of the issue in the U.S. context. In FY2018, the number of IPR seizures at the U.S. border totaled 33,810 commodities (shipped by express, mail, cargo, and other ways) valued at $1.4 billion (manufacturer's suggested retail price, MSRP).39 The total number of seizures per year has been increasing while the estimated value remained relatively constant since 2014 (Figure 3).
|
Figure 3. Overview of IPR Seizures by CBP FY2010-FY2018 |
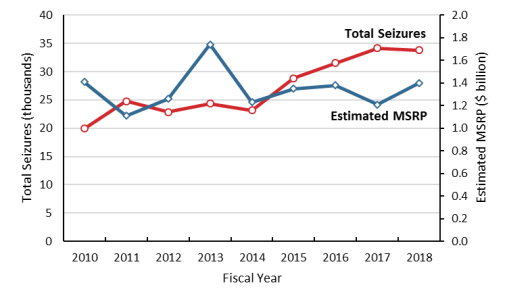 |
|
Source: U.S. Customs and Border Protection, IPR annual seizure statistics. |
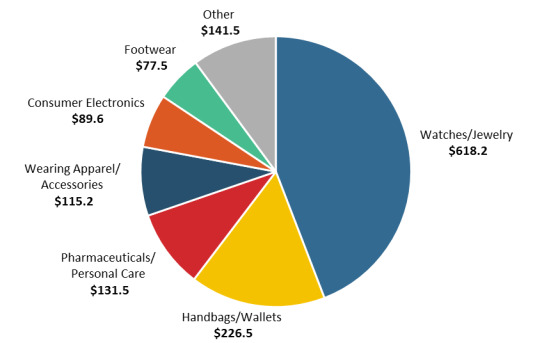
China and Hong Kong ranked as the two largest source economies for seizures by value (see
Table 2). The commodities seized were diverse, with watches/jewelry and handbags/wallets being the top two types seized. Goods seized in FY2018 included shipments of circumvention devices that violated the Digital Millennium Copyright Act (DMCA, P.L. 105-304). Customs data may be limited in that they do not reflect digital-based IPR infringement.
Table 2. IPR Seizures at U.S. Borders: Source Economies, FY2018
(Estimated MSRP, millions of U.S. dollars)
|
U.S. Trading Partner |
Estimated MSRP |
% of Total |
|
Total |
$1,399.9 |
100% |
|
China |
$761.1 |
54.0% |
|
Hong Kong |
$440.3 |
31.0% |
|
India |
$20.0 |
1.0% |
|
Korea |
$10.1 |
0.7% |
|
Canada |
$7.8 |
0.6% |
|
Turkey |
$5.8 |
0.4% |
|
Vietnam |
$5.2 |
0.4% |
|
Taiwan |
$5.0 |
0.4% |
|
Malaysia |
$4.7 |
0.3% |
|
Pakistan |
$2.8 |
0.2% |
|
All Others |
$137.1 |
10% |
Source: CRS analysis of data from Department of Homeland Security, "Intellectual Property Rights Seizure Statistics Fiscal Year 2018."
Notes: Based on manufacturer's suggested retail price (MSRP) of goods had they been genuine.
Overall U.S. Estimates
U.S. industries that rely on IPR protection claim to lose billions of dollars in revenue annually due to piracy and counterfeiting. Beyond these direct losses, the United States may face additional "downstream" losses from counterfeiting and piracy. IPR infringement could result in the loss of jobs that would have been created if the infringement did not occur, which could translate into lost earnings by U.S. workers and, in turn, lost tax revenues for federal, state, and local governments.40 Attempts have been made in specific economic sectors to quantify the IPR infringement levels and related losses to legitimate U.S. businesses.
A private Commission on the Theft of American Intellectual Property estimates the total level of U.S. economic losses to international theft of U.S. IP to be hundreds of billions dollars per year. In 2017, the commission estimated the annual cost to the U.S. economy due to counterfeit goods, pirated software, and theft of trade secrets to be between $225 billion and $600 billion; this estimate does not include the costs of patent infringement and economic espionage because they are difficult to quantify.41 These estimates have been cited widely, including in the annual IP report to Congress by the U.S. Intellectual Property Enforcement Coordinator (IPEC), a statutorily created position in the White House (P.L. 110-403).42 Efforts also have been made to quantify U.S. economic losses from IPR infringement in terms of specific countries (see text box).
|
Estimate of Losses to U.S. Firms from IPR Infringement in China The U.S. International Trade Commission (ITC) estimated losses to "firms in the U.S. IP-intensive economy that conducted business in China in 2009" to be about $48.2 billion in sales, royalties, or license fees due to IPR infringement in China. According to the ITC, this estimate is based on statistical analysis that falls within a broad range of $14.2 billion to $90.5 billion; the range reflects limitations of the underlying data as many firms were unable to calculate losses. In terms of specific sectors, the information/other services sector sustained the largest losses—at a point estimate of $26.7 billion, within a range of $11.8 billion to $48.9 billion. In terms of specific types of IPR infringement, losses from copyright infringement were the largest—at a point estimate of $23.7 billion, within a range of $10.2 billion to $37.3 billion. ITC also estimated that firms in the U.S. IP-intensive economy spent about $4.8 billion (within a range of $279.1 million to $9.4 billion) in 2009 to address possible Chinese IPR infringement. According to submissions from stakeholders to the 2018 U.S. Trade Representative (USTR) Section 301 report on China, there are also intangible losses to U.S. firms from IPR infringement. For example, technology transfer requirements when U.S. firms want to invest in the Chinese market may make U.S. firms less competitive in the global market when they lose exclusive rights to their IP. In its submission to USTR for the purpose of the Section 301 report, a U.S. firm estimated that it sustained "more than $120 million in damages in the form of lost sales and revenue" as a result of Chinese state-sponsored cyber theft. The firm further stated that it lost its first-mover advantage and competitiveness in the market. Source: ITC, China: Effects of Intellectual Property Infringement and Indigenous Innovation Policies on the U.S. Economy, Investigation No. 332-519, USITC Publication 4226, May 2011; USTR, Findings of the Investigation into China's Acts, Policies, Practices Related to Technology Transfer, Intellectual Property, and Innovation Under Section 301 of the Trade Act of 1974, March 22, 2018. Note: ITC results reflect responses to an ITC questionnaire to 5,051 U.S. firms in sectors considered to be IP-intensive. ITC used statistical sampling techniques to extrapolate results to the U.S. IP-intensive economy (16.3% of the U.S. economy). The statistical significance of the findings varied. See the report for more information. |
In terms of losses from cyber theft of IP, a 2018 report by McAfee and the Center for Strategic and International Studies (CSIS) estimates annual losses to be $10 billion to $12 billion in the United States and $50 billion to $60 billion globally.43
The Organizational Structure of IPR Protection
Given the importance of intellectual property to the U.S. economy and the economic losses associated with counterfeiting and piracy, the United States is a leading advocate of strong global IPR rules. Since the mid-1980s, the United States has integrated IPR policy in its international trade policy activities, pursuing enhanced IPR laws and enforcement through multilateral, regional and bilateral trade agreements, and national trade laws.
Multilateral IPR System
World Trade Organization (WTO)
At the center of the present multilateral trading system is the World Trade Organization, an international organization established in 1995 as the successor to the General Agreements on Tariffs and Trade (GATT).44 The WTO was established as the result of the Uruguay Round of multilateral trade negotiations (1986-1994), which led to agreements to liberalize and establish or enhance rules on trade in goods, services, agriculture, and other nontariff barriers to trade. One of the Uruguay Round agreements was the Agreement on Trade-Related Aspects of Intellectual Property Rights, which sets minimum standards on IPR protection and enforcement with which all WTO member states must comply. The United States, European countries, and the IPR business community were instrumental in including IPR on the Uruguay Round agenda. Many developing countries were wary of including IPR in trade negotiations, preferring to discuss treatment of IP under the World Intellectual Property Organization (see below) instead. However, developing countries agreed to address IP issues in the WTO after being granted delayed compliance periods, and after achieving negotiating goals on other issues, such as the end of quotas on textiles and clothing.
While previous international treaties on IPR continue to exist, the TRIPS Agreement was the first time that intellectual property rules were incorporated into the multilateral trading system. Two basic tenets of the TRIPS Agreement are national treatment (signatories must treat nationals of other WTO members no less favorably in terms of IPR protection than the country's own nationals) and most-favored-nation treatment (any advantage in IPR protection granted to nationals of another WTO member shall be granted to nationals of all other WTO member states).
Much of the TRIPS Agreement sets out the extent of the agreement's coverage of the various types of intellectual property: patents, copyrights, trademarks, trade secrets, GIs, industrial designs, layout of circuitry design, and test data. The TRIPS Agreement provisions build on several existing IPR treaties administered by the WIPO (discussed below). Another part of the TRIPS Agreement provides standards of enforcement for IPR covered by the agreement. It enumerates standards for civil and administrative procedures and remedies, the application of border measures, and criminal procedures. A Council for the TRIPS Agreement was established to monitor implementation of the agreement and transitional arrangements were devised for developing countries. Finally, the agreement provides for the resolution of disputes under the Uruguay Round Agreement's Dispute Settlement Understanding (see text box). The binding nature of the WTO dispute settlement mechanism, with the possibility of the withdrawal of trade concessions (usually the reimposition of tariffs) for noncompliance, sets this agreement apart from previous IPR treaties that did not have effective dispute settlement mechanisms.
|
U.S. WTO Cases Against China on IPR The United States has filed three cases against China regarding IP: two that challenged Chinese practices under the TRIPS Agreement and one challenge under the General Agreement on Trade in Services (GATS). The first two were brought under the George W. Bush Administration and the third under President Trump. DS 362: Measures Affecting the Protection and Enforcement of Intellectual Property Rights. In this case brought in 2007, the dispute settlement panel largely ruled in the favor of the United States that
DS542: Certain Measures Concerning the Protection of Intellectual Property Rights. In this case, the United States alleges that China allows domestic firms to continue to use patented technology after a licensing contract ends and requires contracts that discriminate against foreign technology. Consultations were requested in March 2018, and a panel was composed in January 2019. However, the case has been suspended since June 11, 2019, to allow for continued consultations between the United States and China. The United States and China signed a phase one trade agreement on January 15, 2020, to resolve some issues raised by the United States under Section 301 of the Trade Act of 1974. Among other things, China committed to strengthen IP enforcement, but most U.S. concerns on IP, technology transfers, and other issues remain to be addressed in a potential phase two deal.45 |
The TRIPS Agreement also seeks a balance of rights and obligations between protecting private right holders and the obligation "to secure social and cultural development that benefits all."46 Article 7 declares that
... the protection and enforcement of IPR should contribute to the promotion of technological innovation and to the transfer and dissemination of technology, to the mutual advantage of producers and users of technological knowledge and in a manner conducive to social and economic welfare and to a balance of rights and obligations.
This paragraph attempts to link the protection of IPR with greater technology transfer, including technology covered by IPR protection, to the developing world. The language itself has been interpreted in various ways. Developed countries have tended to consider this language exhortatory, but developing countries have tried, without much success, to make technology transfer a meaningful obligation within the TRIPS Agreement system. Article 66.2 of the agreement requires developed country members to provide incentives to their enterprises and institutions to promote technology transfer to least-developed countries (LDCs) to assist them in establishing a viable technology base. Developed countries report annually on their efforts to encourage technology transfer.
Complying with international IPR standards may impose greater burdens on developing countries than developed countries. Developing countries generally have to engage in greater efforts to bring their laws, judicial processes, and enforcement mechanisms into compliance with the TRIPS Agreement. Consequently, developing countries were given an extended period of time in which to bring their laws and enforcement mechanisms into compliance with the TRIPS Agreement. Developing countries and post-Soviet states were given an additional four years from the entry into force of the agreement (January 1, 1995). For products that were not covered by a country's patent system (such as pharmaceuticals in many cases), an additional five years was granted to bring such products under coverage. For developing countries, all provisions of the TRIPS agreement should now be in force. For the least developed countries, the phase-in period for IPR commitments was originally extended 10 years to January 1, 2006 (Article 66.1). In 2002, the WTO extended IPR obligations for LDCs with respect to pharmaceuticals to January 1, 2016.47 In addition, the WTO has extended the overall transitional period twice for LDCs.48 As such, LDCs are not required to apply TRIPS Agreement provisions—other than Articles 3, 4, and 5, until July 1, 2021, or until they cease to be LDCs.49 Article 66.1 acknowledges the:
special needs and requirements of least-developed country Members, their economic, financial and administrative constraints, and their need for flexibility to create a viable technological base.
Doha Declaration on the TRIPS Agreement and Public Health
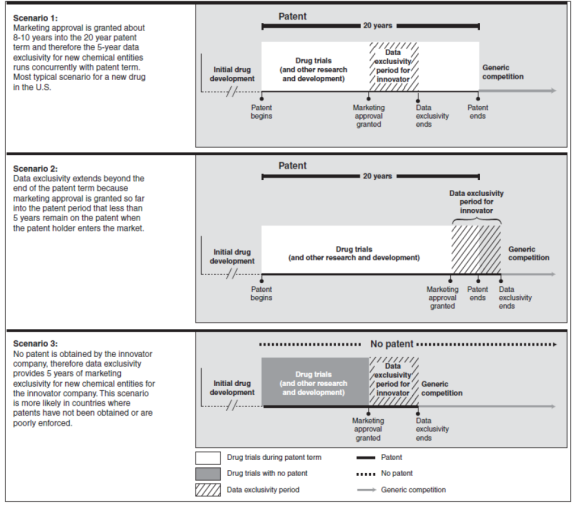
In agreeing to launch the Doha Round of WTO trade negotiations, trade ministers adopted a "Declaration on the TRIPS Agreement and Public Health" on November 14, 2001.50 The Declaration sought to alleviate developing country dissatisfaction with aspects of the TRIPS regime. It delayed the implementation of patent system provisions for pharmaceutical products for LDCs until 2016. The declaration committed member states to interpret and implement the agreement to support public health and to promote access to medicines for all. The Declaration recognized certain "flexibilities" in the TRIPS Agreement to allow each member to grant compulsory licenses for pharmaceuticals and to determine what constitutes a national emergency, expressly including public health emergencies such as HIV/AIDS, malaria, and tuberculosis or other epidemics. Paragraph 6 of the Doha Declaration directed the WTO members to formulate a solution to a related concern, the use of compulsory licensing by countries with insufficient or inadequate manufacturing capability. (See COVID-19 text box below.)
On the eve of the Cancun Ministerial in August 2003, WTO members agreed on a Decision51 to waive the domestic market provision of the TRIPS article on compulsory licensing (Article 31(f)) for exports of pharmaceutical products for "HIV/AIDS, malaria, tuberculosis and other epidemics" to LDCs and countries with insufficient manufacturing capacity. This Decision was incorporated as an amendment to the TRIPS agreement at the Hong Kong Ministerial in December 2005.
The amendment required ratification from two-thirds of WTO member states. The deadline for ratification was extended five times before the amendment entered into force on January 23, 2017. To date, 102 of the total 162 WTO members52 have ratified the amendment.53 A group of high-income countries (Australia, Canada, the European Union, Iceland, Japan, New Zealand, Norway, Switzerland, and the United States) declared they would not avail themselves of this option as importers.54
The system established by the WTO allows LDCs and countries without sufficient manufacturing capacity to issue a compulsory license to a company in a country that can produce such a product. After a matching compulsory license is issued by the producer country, the drug can be manufactured and exported subject to various notification requirements, as well as quantity and safeguard restrictions. While several exporting countries have established laws and procedures for implementing this system, one (Rwanda) has availed itself of the system to import HIV/AIDS medicines from a generic manufacturer in Canada.55
|
COVID-19 and Access to Medicine The Coronavirus Disease 2019 (COVID-19) pandemic may reopen a debate over the relationship between WTO trading rules and countries' ability to obtain needed drugs or vaccinations. As noted above, TRIPS created the first enforceable minimum standards for international IPR. It affirmed that patents "shall be available for any inventions…in all fields of technology, provided that they are new, involve an inventive step and are capable of industrial application." It also applied the principle of nondiscrimination on issuance of patents based on technology, place of invention, or site of use. This standard was particularly important to innovative pharmaceutical manufacturers because several countries did not provide for patenting pharmaceutical products prior to TRIPS, or, as in the case of India, provided process patents that covered the manufacturing process but not product itself. However, TRIPS does provide for limited exceptions to the patent right. For example, a country may limit patent rights provided the limitation does not "unreasonably" conflict with the normal exploitation of a patent. The agreement also contains exceptions allowing a party to exclude from patentability items to protect human life and health, as well as diagnostic, therapeutic and surgical measures. The Doha Declaration on TRIPS and Public Health (see above) affirmed that TRIPS provisions should be interpreted to promote public health and access to medicine. TRIPS also allows for compulsory licensing, but places limitations on its use. A compulsory license is an authorization by a government for third parties (such as a company or the government itself) to manufacture or use a product under patent without the permission of the rights holder. TRIPS permits signatories to issue compulsory licenses for patented inventions, if the third party attempts to obtain permission from the patent holder and negotiates reasonable commercial terms, although this requirement can be waived in times of national emergency or other extenuating circumstances. In any case, the third party must provide "adequate" remuneration to the patent holder for the use of the patent. Another restriction limits its use primarily to the domestic market, although countries may issue compulsory license to send products to least-developed countries that lack domestic production capabilities. The allowance for least-developed countries and a clarification of the meaning of national emergency became part of the amendment to TRIPS that originated with the Doha Declaration. U.S. bilateral and regional FTAs largely have not addressed the issue of compulsory licensing, but have contained provisions incorporating the Doha Declaration. In practice, the use of compulsory licenses has been rare; the threat of invoking a compulsory license as a negotiating tactic for countries to obtain better prices from a manufacturer has been more common. The United States generally has sought to dissuade other nations from using compulsory licensing, even placing greater limitations on its use in early U.S. FTAs with Australia, Singapore, and Jordan. However, with the COVID-19 virus, it has been reported that certain governments are taking preliminary steps to revisit its use. Israel is the first country to issue a compulsory license in the context of COVID-19 for the AbbVie drug Kaletra (lopinavir/ ritonavir). The next day AbbVie announced it would no longer enforce patents worldwide for lopinavir/ritonavir.56 In March 2020, the parliaments of Canada and Germany passed legislation clarifying or streamlining the ability to use compulsory licenses in their countries. The National Assemblies of Chile and Ecuador are calling for the use of compulsory licenses in fighting the COVID-19 pandemic.57 For more information see, see CRS Legal Sidebar LSB10436, COVID-19: International Trade and Access to Pharmaceutical Products, by Nina M. Hart. |
World Intellectual Property Organization (WIPO)
In addition to the WTO, the other main multilateral venue for addressing IPR issues is the World Intellectual Property Organization, a specialized agency affiliated with the United Nations, with its own executive, legislative, and budgetary powers. Established in 1970, following the 1967 WIPO Convention's entry into force, WIPO is charged with fostering the effective use and protection of intellectual property globally. WIPO's mandate focuses exclusively on intellectual property, in contrast to the WTO's broader international trade mandate. WIPO's antecedents are the 1883 Paris Convention for the Protection of Industrial Property and the 1886 Berne Convention for the Protection of Literary and Artistic Work. Most of the substantive provisions of these two treaties are incorporated in the WTO's TRIPS Agreement. WIPO's primary function is to administer a group of IPR treaties which put forth minimum standards for member states. All international IPR treaties, save TRIPS, are administered by WIPO.
The Trump Administration has prioritized the need to counter growing Chinese influence in global functional organizations, including WIPO. Its goal is to preserve the integrity of these organizations to ensure they remain impartial and credible and that their focus and work continue to support U.S. interests and key tenets of the open global trading system, including protection of IPR. In On February 26, 2020, China's ambassador to the United Nations Chen Xu accused the United States of meddling in the upcoming World Intellectual Property Organization leadership election. U.S. diplomats reportedly lobbied to block China's candidate, Wang Binying, and promote Daren Tang, a candidate nominated by Singapore. On March 4, 2020, the WIPO Coordination Committee nominated Daren Tang to be the next Director General of WIPO. Mr. Tang prevailed with 55 votes, while Ms. Wang received 28 votes.
To address digital technology issues not dealt with in the TRIPS Agreement, WIPO established the WIPO Copyright Treaty (WCT) and WIPO Performance and Phonograms Treaty (WPPT) in 1996, oftentimes collectively referred to as the "WIPO Internet Treaties." These treaties establish international norms aimed at preventing unauthorized access to and use of creative works on the internet or other digital networks.
Other WIPO activities include patent law harmonization efforts. In 2000, WIPO signatories adopted the Patent Law Treaty (PLT), which called for harmonization of patent procedures. This agreement went into force on April 28, 2005. Discussions began in 2001 for a Substantive Patent Law Treaty (SPLT), which would target harmonization issues specifically related to patent grants, but were put on hold in 2006. Different views reportedly emerged among developed and developing countries on what should be the objectives of substantive harmonization of patent laws, including whether it was an appropriate goal.58 Government leaders participating in the Group of 8 (G-8) meeting in July 2008 called for "accelerated discussions" of the SPLT.59 While discussions remain stalled, the main focus of the WIPO's work in this area has been on "building a technical and legal resource base from which to hold informed discussions in order to develop a work program" on various patent issues.60 Presently, patent law harmonization efforts also are occurring in groupings outside of WIPO, including the Trilateral Cooperation, composed of the European Patent Office, Japan Patent Office, and U.S. Patent and Trademark Office (USPTO); another forum is the IP5, composed of the members of the Trilateral Cooperation and also the Korean Intellectual Property Office and China's State Intellectual Property Office.61
WIPO's other functions include assisting member states through training programs, legislative information, intellectual property institutional development, automation and office modernization efforts, and public awareness activities. WIPO's enforcement activities are more limited than those of the WTO. Through its Advisory Committee on Enforcement (ACE), WIPO cooperates with member states to promote international coordination on enforcement activities.
U.S. Trade Law
Several provisions of U.S. law address IPR trade policy and enforcement. These laws are implemented and administered by a number of U.S. government agencies and coordinating bodies (see Table 4 and Appendix A).
Special 301
Section 301 of the Trade Act of 1974 as amended (P.L. 93-618, 19 U.S.C. §2242) is the principal U.S. statute for identifying foreign trade barriers due to inadequate intellectual property protection. The 1988 Omnibus Trade and Competitiveness Act (P.L. 100-418) strengthened section 301 by creating "Special 301" provisions, which require the USTR to conduct an annual review of foreign countries' intellectual property policies and practices. By April 30 of each year, the USTR must identify countries that do not offer "adequate and effective" protection of IPR or "fair and equitable market access" to U.S. entities that rely on intellectual property rights. According to an amendment to the Special 301 provisions by the Uruguay Round Agreements Act (P.L. 103-465), the USTR can identify a country as denying sufficient intellectual property protection even if the country is complying with its TRIPS commitments. These findings are submitted in the USTR's annual "Special 301" report (see Table 3). Most recently, the Trade Facilitation and Trade Enforcement Act of 2015 (P.L. 114-125) added trade secrets to list of the types of IPR whose protection by a foreign country is subject to monitoring under Special 301.
The USTR can designate countries in one of several statutorily or administratively created categories:
- Priority Foreign Country: A statutory category for those designated by the USTR as having "the most onerous or egregious acts, policies or practices that deny intellectual property protection and limit market access to U.S. persons or firms depending on intellectual property rights protection" with the "greatest adverse impact (actual or potential) on the relevant United States products." These countries may be investigated under section 301 provisions of the Trade Act of 1974.62 If a country is named as a "Priority Foreign Country," the USTR must launch an investigation into that country's IPR practices. The USTR may suspend trade concessions and impose import restrictions or duties, or enter into a binding agreement with the priority country that would eliminate the act, policy, or practice under scrutiny. Since the advent of the WTO, the United States has brought cases to the WTO rather than impose unilateral retaliation.
- Priority Watch List: An administrative category created by the USTR for those countries whose acts, policies, and practices warrant concern, but who do not meet all of the criteria for identification as Priority Foreign Country. The USTR may place a country on the Priority Watch List when the country lacks proper intellectual property protection and has a market of significant U.S. interest. If designated on the Priority Watch List, the USTR must develop an action plan with respect to that foreign country. If the President, in consultation with USTR, determines that the foreign country fails to meet the action plan benchmarks, then the President may take appropriate action with respect to the foreign country.
- Watch List: An administrative category created by USTR to designate countries that have intellectual property protection inadequacies that are less severe than those on the Priority Watch List, but still attract U.S. attention.
- Section 306 Monitoring. A tool used by USTR to monitor countries for compliance with bilateral intellectual property agreements used to resolve investigations under section 301.
- Out-of-Cycle Review. A tool used by USTR to monitor countries' progress on intellectual property issues, and which may result in status changes for the following year's Special 301 report. In 2010, USTR also began publishing annually the Notorious Markets List as an out-of-cycle review separately from the annual Special 301 report; the report identifies online and physical markets "that reportedly engage in, facilitate, turn a blind eye to, or benefit from substantial copyright piracy and trademark counterfeiting."
|
Special 301 Category |
2020 Special 301 Designation |
|
Priority Foreign Country |
|
|
Priority Watch List |
Algeria, Argentina, Chile, China, India, Indonesia, Russia, Saudi Arabia, Ukraine, and Venezuela |
|
Watch List |
Barbados, Bolivia, Brazil, Canada, Colombia, Dominican Republic, Ecuador, Egypt, Guatemala, Kuwait, Lebanon, Mexico, Pakistan, Paraguay, Peru, Romania, Thailand, Trinidad & Tobago, Turkey, Turkmenistan, United Arab Emirates, Uzbekistan, and Vietnam |
|
Section 306 Monitoring |
China |
|
Out-of-Cycle Reviews |
|
Source: CRS adaption from USTR, 2020 Special 301 Report.
Notes: For the 2020 Special 301 Report, USTR reviewed the IPR policies and practices of more than 100 countries, and designated 36 of the countries in one of several categories.
The Special 301 statute provides the overall guideline for identifying countries for the various lists. However, placement on one of the lists takes into consideration a host of factors specific to the country, including the level and scope of the country's IPR infringement and their impact on the U.S. economy, the strength of the country's IPR laws and the effectiveness of their enforcement, progress made by the country in improving IPR protection and enforcement in the past year, and the sincerity of the country's commitment to multilateral and bilateral trade agreements. No "weighting criteria" or formula exists to determine the placement of a country on the watch list. Furthermore, no particular threshold exists for determining when a country should be upgraded or downgraded on the list. In making determinations, the USTR gathers information based on its annual trade barriers reports, as well as consultations with a wide variety of sources, including industry groups, other private sector representative, Congress, and foreign governments.
Section 301
Title III of the Tariff Act of 1930, as amended (Sections 301 through 310, 19 U.S.C. §2411)—collectively referred to as "Section 301"—grants the USTR a range of responsibilities and authorities to investigate and take action to enforce U.S. rights under trade agreements and respond to certain foreign trade practices.63 Section 301 provides a statutory means by which the United States imposes trade sanctions on foreign countries that violate U.S. trade agreements or engage in acts that are "unjustifiable" or "unreasonable" and burden U.S. commerce. Prior to 1995, the United States used Section 301 extensively to pressure other countries to eliminate trade barriers and open markets to U.S. exports. The creation of an enforceable dispute settlement mechanism in the WTO significantly reduced U.S. use of Section 301. The United States retains the flexibility to determine whether to seek recourse for foreign unfair trade practices in the WTO and/or act unilaterally. President Trump has been more willing to act unilaterally to promote what the Administration considers to be "free," "fair," and "reciprocal" trade. The President has imposed increased tariffs under Section 301 on U.S. imports from China due to concerns over China's forced technology transfer requirements and intellectual property rights practices, including cyber-enabled theft of U.S. IPR and trade secrets.64 The Phase I trade deal that the Trump Administration reached with the Chinese government addresses some aspects of IP issues, while leaving other systemic IP issues to address in potential bilateral trade talks.65
Section 337
Section 337 of the Tariff Act of 1930, as amended (19 U.S.C. §1337), prohibits unfair methods of competition or other unfair acts in the importation of products into the United States. It also prohibits the importation of articles that infringe valid U.S. patents, copyrights, processes, trademarks, semiconductor products produced by infringing a protected mask work (e.g., integrated circuit designs), or protected design rights. While the statute has been used to counter imports of products judged to be produced by unfair competition, monopolistic, or anti-competitive practices, in recent years it has become increasingly used for its IPR enforcement functions. Under the statute, the import or sale of an infringing product is illegal only if a U.S. industry is producing an article covered by the relevant IPR or is in the process of being established. Unlike other trade remedies, such as antidumping or countervailing duty actions, no showing of injury due to the import is required for "statutory" IP cases.
The U.S. International Trade Commission (ITC) administers Section 337 proceedings. ITC investigates complaints either brought to it, mainly by companies, or ones commenced under its own initiative. An administrative law judge provides an initial determination to the ITC which can accept the initial determination or order a further review of it in whole or in part. If the ITC finds a violation, it may issue two types of remedies: exclusion orders or cease and desist orders.
- Exclusion orders, enforced by the U.S. Customs and Border Protection (CBP), are issued to stop infringing imports from entering the United States. Exclusion orders can be general or limited. General exclusion orders apply to all products that are found in violation of Section 337, regardless of source. Limited exclusion orders apply to the goods originating from the specific firm(s) found to be in violation of Section 337. Limited exclusion orders typically are the more commonly issued type of exclusion order. The ITC issues general exclusion orders if such a broad-based exclusion is necessary to prevent the circumvention of the limited exclusion order, or if there is a pattern of violation and it is difficult to identify the source of infringing products.
- Cease and desist orders, enforced by ITC, require the firm to stop the sale of the infringing product in the United States.
The ITC may consider several public interest criteria and decline to issue a remedy. Also, the President may disapprove a remedial order during a 60-day review period for "policy reasons." A presidential review of a remedial order often considers several relevant factors, including "(1) public health and welfare; (2) competitive conditions in the U.S. economy; (3) production of competitive articles in the United States; (4) U.S. consumers; and (5) U.S. foreign relations, economic and political."66
The number of active Section 337 investigations conducted by the ITC generally has trended upward over the past decade (see text box). The overwhelming majority of Section 337 cases involve allegations by private firms of patent infringement. Investigations concern a range of technologies, including smartphones and other wireless devices, smart televisions, semiconductors, GPS devices, windshield wiper blades, and tires.67 According to the ITC, there is "substantial overlap between the industries that dominate our IP docket and the four industries determined in a Department of Commerce study to be the most patent-intensive industries in the United States"—computer and peripheral equipment, communications equipment, semiconductor and other electronic components, and other computer and electronic products.68
|
FY2019 Section 337 Statistics Number of new complaints and ancillary proceedings – 58 (compared to 40 in FY2006) Number of investigations and ancillary proceedings completed – 60 (compared to 30 in FY2006) Number of active investigations: 127 in FY2019 (compared to 70 in FY2006) Types of unfair acts alleged in active investigations: sole patent infringement – 110; solely trademark infringement – 3; solely trade secret misappropriation – 4; combination of unfair acts alleged - 10 Number of investigations completed on the merits: 22 (compared to 12 in FY2006) Length of investigations completed on the merits: shortest – 9.4 months, longest – 29.3 months, average -17.7 months (compared to, in FY2006, shortest – 3.5 months, longest 19.0 months, and average – 12.0 months) Number of active exclusion orders (as of December 31, 2018): 114 Number of remedial orders issued: general exclusion orders - 5, limited exclusion orders - 10, cease and desist orders – 16 (compared to, in FY2006, GEOs – 3, LEOs -5, CDOs – 2) Settlement/consent order share of total number of investigations terminated – 33% of 42 investigations (compared to 46% of 26 investigations in FY2006) Complaints withdraw share of total number of investigations terminated – 12% of 42 investigations (compared to 8% of 26 investigations in FY2006) Source: U.S. International Trade Commission. |
Legislative efforts related to Section 337 have focused on addressing jurisdictional problems associated with holding foreign websites accountable for piracy and counterfeiting, renewing congressional and public debate about the balance between protecting U.S. intellectual property and promoting innovation.69 Congress could take these issues up again, as well as other issues, including the effectiveness of CBP's enforcement of Section 337 exclusion orders. A 2014 Government Accountability Office study found that CBP's management of its exclusion order process at ports contained weaknesses that result in inefficiencies and an increased risk of infringing products entering U.S. commerce; it recommended that CBP update its internal guidance related to sharing information sharing for trade alerts and monitoring.70 CBP has since implemented recommendations to ensure that active exclusion orders from the ITC are posted on CBP's intranet.71
Generalized System of Preferences
The Generalized System of Preferences (GSP) is a U.S. trade and development program that provides preferential duty-free entry to certain products from designated developing countries.72 The purpose of the program is to foster economic growth in developing countries by increasing their export markets. GSP operates on a nonreciprocal basis. The Trade Act of 1974, as amended (19 U.S.C. §2461-67), authorized the GSP for a ten-year timeframe, and the program has been renewed from time to time. Congress most recently extended the GSP program until December 31, 2020, in the Consolidated Appropriations Act, 2018 (P.L. 115-141).
Although GSP is nonreciprocal, it can be used to promote stronger intellectual property protection and enforcement abroad. Under the GSP statute, the President must consider a set of mandatory criteria that a country must fulfill in order to be designated as a GSP beneficiary. Additionally, the President may evaluate a country on the basis of certain discretionary criteria, including the country's provision of IPR protection.73 For example, in light of heightened concern over India's intellectual property environment, President Trump removed India from the Generalized System of Preferences beneficiary list on May 31, 2019.74
The GSP program undergoes an annual review by the GSP Subcommittee of the interagency Trade Policy Staff Committee (TPSC), which is headed by the USTR. As part of its evaluation, the TPSC addresses concerns about specific country practices (such as intellectual property protection) and makes recommendations to the President. In October 2019, the President partially restored GSP benefits to Ukraine for certain products based on the determination that the country made progress towards providing IPR protection; Ukraine's GSP benefits had been suspended in December 2017.75 Based on industry petitions concerning IPR protection, USTR reports as ongoing its reviews of the country practices of Indonesia, South Africa, and Uzbekistan.76
|
Department of Commerce |
Department of Homeland Security |
Department of Justice |
Other Federal Agencies |
Coordinating and Advisory Bodies |
|
Patent and Trademark Office International Trade Administration |
Customs and Border Protection Immigration and Customs Enforcement U.S. Secret Service |
Civil Division Criminal Division Federal Bureau of Investigation Office of Justice Program U.S. Attorney's Office |
U.S. Trade Representative Department of Health and Human Services (Food and Drug Administration) Library of Congress (Copyright Office) Department of State U.S. Agency for International Development U.S. International Trade Commission |
Office of the U.S. Intellectual Property Enforcement Coordinator (IPEC) National Intellectual Property Rights Coordination Center (IPR Center) Interagency for Trade Implementation, Monitoring, and Enforcement (ICTIME) Private Sector Advisory Committee System |
Source: CRS analysis.
Notes: For more information, see Appendix A.
U.S. Trade Promotion Authority and Negotiating Objectives
Trade promotion authority (TPA) is the time-limited authority that Congress uses to set U.S. trade negotiating objectives, to establish notification and consultation requirements, and to have implementing bills for certain reciprocal trade agreements considered under expedited procedures, provided certain statutory requirements are met.77 In recent grants of TPA, IPR issues have become important negotiating objectives.
IPR negotiating objectives for FTAs were first enacted by the Omnibus Trade and Competitiveness Act of 1988 (P.L. 100-418). The statute sought enactment and enforcement of adequate IPR protection from negotiating partners. It also sought to strengthen international rules, dispute settlement, and enforcement procedures through the General Agreement on Tariffs and Trade and other existing intellectual property conventions. This negotiating mandate led to the establishment of the TRIPS Agreement during the Uruguay Round of multilateral trade liberalization negotiations and the IPR provisions in the North American Free Trade Agreement. In the period since the 1988 Act, the IPR provisions of NAFTA and the TRIPS Agreement became the template for other bilateral or regional FTAs. The focus of IPR negotiating objectives shifted from creating to strengthening the IPR trade regime with the Trade Promotion Authority Act of 2002 (P.L. 107-210), under which several FTA negotiations were concluded by the George W. Bush Administration.
2002 Trade Promotion Authority
The IPR negotiating objectives in the 2002 TPA were highly significant to the future contours of U.S. FTA negotiations. The objective to negotiate trade agreements IPR terms that "reflect a standard of protection similar to that found in U.S. law" led to the negotiation of provisions that go beyond the level of protection provided in the WTO TRIPS Agreement. Often referred to as "TRIPS-plus" provisions, they include expanding IPR to new sectors, establishing more extensive standards of protection, and reducing the flexibility options available in TRIPS, such as with respect to compulsory licensing. Some of the new measures also address technological innovations that have come about since the TRIPS Agreement.
The objective to apply existing IPR protections to digital media reflected the changing nature of global commerce. The language sought to extend provisions for IPR protection to new and emerging technologies and methods of transmission and dissemination. The language also called for standards of enforcement to keep pace with technological change and allow right holders legal and technological protections for their works over the internet and other new media.
May 10, 2007 Bipartisan Trade Agreement
The May 10, 2007 Bipartisan Trade Agreement ("May 10 Agreement")—related to the then-pending FTAs with Colombia, Panama, Peru, and South Korea—established certain flexibilities for patent protections to promote further access to medicines in developing countries while maintaining a strong overall level of IPR protection.78 After the transfer of control of the House following the 2006 elections, some Members of the new Democratic majority sought certain changes in these pending U.S. FTAs. With respect to IPR, the congressional leadership sought to ensure that pending FTAs allowed developing country trading partners enough flexibility both to meet their IPR obligations and to promote access to life-saving medicines. A Bipartisan Trade Agreement between the Bush Administration and the House leadership, building on the 2002 TPA negotiating objectives, was reached on May 10, 2007.79 Following the Agreement, IPR language previously negotiated in the FTAs with Peru, Panama, and Colombia was modified to reflect its principles. The U.S.-South Korea FTA (KORUS) was not modified because the United States considers South Korea to be a developed country.
2015 Trade Promotion Authority
Congress passed the Bipartisan Comprehensive Trade Promotion and Accountability Act (P.L. 114-26) (TPA-2015) in June 2015, and President Obama signed the legislation on June 29, 2015. The IPR negotiating objectives include and expand on the 2002 objectives. The 2015 objectives recognize the importance of digital trade to the economy and seek provisions to prohibit cyber- and trade secret theft. The IPR objectives are considered principal negotiating objectives. This means that a procedural disapproval resolution could be introduced to strip FTA implementing legislation of expedited legislation procedures if the legislation fails "to make progress on the policies, priorities, and objectives of the Act."80 The objectives include
- Furthering adequate and effective protection of IPR through accelerated full implementation of the TRIPS Agreement and by ensuring FTAs negotiated by the United States "reflect a standard of [IPR] protection similar to that found in U.S. law";
- Protecting IPR related to new technologies and new methods of transmission and distribution in a manner that "facilitates legitimate trade";
- Eliminating discriminatory treatment in the use and enforcement of IPR;
- Ensuring adequate rights holder protection through digital rights management practices;
- Providing for strong enforcement of IPR;
- Negotiating the prevention and elimination of government involvement in violations of IPR such as cyber-theft or piracy;81 and
- Reaffirming the Doha Declaration on the TRIPS Agreement and Public Health, with additional language to "ensure that trade agreements foster innovation and access to medicine."82
Free Trade Agreements and Negotiations under the Trump Administration
In recent years, the United States increasingly has focused on free trade agreements (FTAs) as an instrument to promote stronger IPR regimes by foreign trading partners. IPR chapters in trade agreements include provisions on patents, copyrights, trademarks, trade secrets, GIs, and enforcement. In general, the United States has viewed the TRIPS Agreement and WIPO-administered treaties as a minimum standard and has pursued higher IPR protection and enforcement levels through regional and bilateral FTAs. To date, the United States has entered into 14 FTAs with 20 countries.
United States-Mexico-Canada Agreement (USMCA)
|
IPR Highlights in USMCA Digital enforcement. Extends IPR enforcement, including for copyrights, to the digital environment. Trade secrets. Requires criminal procedures and penalties for trade secret theft, including cyber-theft; also clarifies that state-owned enterprises are subject to trade secret protection requirements. Internet Service Providers (ISPs). Requires "notice and takedown" processes to address ISP liability while allowing an alternative system to remain for Canada ("notice and notice"). Trademarks. Extends trademark protection to sounds and "collective marks"; removes administrative requirements to enable easier protection and enforcement of trademarks. Geographical indications (GIs). Requires administrative procedures for recognizing and opposing GIs, including guidelines for determining when a name is common. Also, for GIs protected through international agreements, includes requirements on transparency and opportunity to comment or oppose GI recognition. |
USMCA is the first trade agreement approved by Congress under the 2015 TPA. In many ways it builds on previous U.S. FTAs, including NAFTA, but it features some divergences from previous FTAs as well. NAFTA was the first FTA to contain an IPR chapter, which in turn was the model for the TRIPS Agreement that came into effect a year later in 1995.83 NAFTA predated the widespread use of the commercial internet, and subsequent IPR chapters in U.S. FTAs contain obligations more extensive than those found in TRIPS and NAFTA.
In general, U.S. FTAs have followed the TPA negotiating objective that agreements should "reflect a standard of protection similar to that found in U.S. law." In addition, President Trump's objectives for the NAFTA renegotiation reflected TPA-2015 and the aims of U.S. negotiators in the Trans-Pacific Partnership, although in some instances the negotiated TPP outcomes were less extensive.84 The United States achieved most of what it sought in the proposed USMCA; however, the Administration and some Members of Congress subsequently negotiated several changes to the agreement, including in the IPR chapter. USMCA changes, and the amendments known as the Protocol of Amendment (POA), are included in the description of core IPR provisions discussed further below. USMCA is currently scheduled to come into effect on July 1, 2020, but the ability to achieve the measures necessary to come into compliance with the accord have cast that timeframe into doubt.
Ongoing and Future Free Trade Agreement Negotiations
IPR issues may arise in a number of ongoing and future U.S. FTA negotiations under the Trump Administration. It remains to be seen to what extent elements of USMCA will serve as a template for these negotiations.
On October 16, 2018, the Trump Administration notified Congress, under TPA, of its intent to enter trade agreement negotiations with the EU, the UK, and Japan.
Regarding the EU, the TPA notification followed the July 2018 Joint Statement (agreed between President Trump and then-European Commission President Jean-Claude Juncker) that aimed to de-escalate trade tensions, including over tariff measures. The negotiations appear to be at an impasse due to lack of U.S.-EU agreement over their scope. While the U.S. specific negotiating objectives envision a broad-based trade agreement, the EU negotiating mandate is limited to non-agricultural tariffs and some regulatory cooperation. The U.S.-EU negotiating approach remains unclear, including the extent to which the negotiations may address IP issues.
The United States and EU both maintain strong IPR standards and generally prioritize IPR protection and enforcement as a key trade negotiating objective. In past U.S.-EU trade negotiations on the proposed Transatlantic Trade and Investment Partnership (T-TIP) under the Obama Administration, treatment of IPR was a major point of debate.85 A key issue was, and continues to be, differing approaches to protection and enforcement of geographic indications. The EU seeks strong GI protection because of GIs' commercial value to EU producers (e.g., Parmesan cheese, Parma ham, Feta cheese, and Champagne). The United States tends to protect GIs through trademark law—as opposed to a separate system—and expresses concern that the EU approach to GIs is "over-broad" and negatively affects trademarks and market access for U.S. products that use generic names.86 Despite these differences, the United States and EU have potential for cooperation on other IP issues, such as developing rules on trade secrets, an area of U.S. and EU concern in light of increased instances of trade secret cyber-theft.87
Similar issues could arise in prospective U.S.-UK trade negotiations, particularly to the extent that the UK remains aligned with EU rules and regulations. GI issues, while potentially significant, may not be as charged as in the U.S.-EU trade negotiations. A major issue for the UK is the potential impact of an FTA on pharmaceutical drug pricing. According to the specific negotiating objectives issues issued by the USTR, a U.S. priority for the negotiations is to "[s]eek standards to ensure that government regulatory reimbursement regimes are transparent, provide procedural fairness, are nondiscriminatory, and provide full market access for U.S. products."88 In the UK, there have been many public calls for ensuring that the National Health Service's pharmaceutical pricing model is not undermined by any IP or regulatory commitments in a U.S.-UK FTA.
In the case of Japan, the scope of specific negotiating objectives released by the USTR include IPR as part of a broad range of issues to be covered in an agreement. However, the initial stage one trade agreement reached by the United States and Japan, which entered into force on January 1, 2020, is limited to industrial and agricultural goods, and cross-border digital trade.89 It is unclear if a second stage of the trade agreement would include IPR issues.
In other developments, on February 6, 2020, President Trump announced that the Administration intends to enter into FTA negotiations with Kenya, and the Administration provided Congress with a formal notification under TPA on March 17, 2020.90 USTR has identified copyright piracy and government use of unauthorized software as issues of concern with respect to Kenya.91
Core Provisions in U.S. Trade Agreements
What follows is a discussion of some of the central patent, copyright, trademark and other IP commitments in U.S. FTAs and how they relate to the WTO TRIPS Agreement (see "World Trade Organization (WTO)").
Patents
Patent protection is one of the more contentious areas of U.S. FTA negotiations on IPR issues. In the context of pharmaceuticals, the United States and other developed countries generally support strong patent rights as necessary to provide incentives for innovation and enable rights holders to recoup R&D and regulatory costs and invest in future innovations. Some developing countries, however, maintain that patents may raise the costs of drugs and delay the entry of lower-cost generic competitors into the market, leading to concerns about affordability and access to medicines.
Many FTAs in force include TRIPS-plus patent provisions, the most prominent of which are patent term length extensions, linkages between regulatory authority and patent rights, data protection, compulsory licensing, and parallel importation. The U.S. FTAs with Peru, Panama, and Colombia respond to the concerns of some Members of Congress over provisions that could restrict access to medicines in these countries and contain less ambitious standards for pharmaceutical patents, compared to previously negotiated FTAs.
Some key patent provisions in U.S. FTAs and their evolution are discussed below.92
Patent-Eligible Subject Matter
TRIPS, NAFTA, subsequent U.S. FTAs, and the USMCA have made patents available "for any inventions, whether products or processes, in all fields of technology, provided that they are new, involve an inventive step and are capable of industrial application."93 These agreements generally have also described three exceptions for which a party can exclude from patentability:
- inventions, the prevention of commercial exploitation within their territory of which is necessary to protect ordre public or morality, including to protect human, animal or plant life or health or to avoid serious prejudice to the environment, provided that such exclusion is not made merely because the exploitation is prohibited by their law;
- diagnostic, therapeutic, and surgical methods for the treatment of humans or animals;
- animals other than microorganisms, and essentially biological processes for the production of plants or animals, other than non-biological and microbiological processes.94
Most agreements require patents be made available for plant varieties, but some allowed the exclusion of plants other than microorganisms.
Since the 2005 U.S.-Morocco FTA, some agreements have required patent coverage of "new uses or methods of using a known product." This protection was also included in the Bahrain, Oman, and South Korea FTAs, as well as the USMCA as originally negotiated. The FTAs with Morocco, Bahrain and Oman also included required patent eligibility for treatments for medical conditions. However, with respect to the final USMCA, the entire provision was dropped in the protocol of amendment (POA). According to House Ways and Means Committee Democrats, the provision would have "locked in the practice of 'patent evergreening' in which pharmaceutical companies obtain hundreds of patents related to a product to block generic competition and price reductions."95 Views are mixed on patent evergreening, as another view is that the practice provides patents for new uses and methods of existing products and incentivizes innovations in developing products with new methods of dispensation (such as that would avoid a trip to the hospital), or a product with fewer side-effects.96
|
Pharmaceutical Patent Protection in India Since 2012, India has denied or revoked patents for several cancer and hepatitis C drugs developed by Western pharmaceutical companies. India's Supreme Court has decided to prohibit patents for certain chemical forms absent a showing of "enhanced efficacy," although the products are protected by patents in many other countries. Innovator companies often seek patents of modified versions of originally patented products, a practice sometimes critically referred to as "evergreening." India's patent laws are designed to discourage evergreening by denying a patent unless there is a showing of enhanced efficacy of the reformulated pharmaceutical product. USTR argues that patents are appropriate because modifications can provide new benefits, such as "fewer side effects, decreased toxicity, improved delivery systems, or temperature or storage stability."97 India also has issued, or threatened to issue, compulsory licenses for pharmaceuticals. For example, in March 2012, the Indian government issued a compulsory license to an Indian pharmaceutical company to produce a generic version of Nexavar, a kidney cancer drug produced by Bayer. India defended its decision on the basis that the price for the patented drug was too high for most Indians.98 According to the 2019 Special 301 Report, U.S. companies operating in India continue to be concerned about the potential threat of compulsory licenses and patent revocation, as well as what they perceive to be overly broad criteria for these actions under India's domestic law. |
Term Adjustment for Unreasonable Granting Authority Delays
An adjustment to the patent term beyond its 20-year protection period may be provided in cases of "unreasonable delays" by patent-granting authorities (e.g., PTO) in issuing patents during the administrative review of patent applications (patent examination). Such extensions increase the length of time right holders have no generic competition, enhancing their ability to recoup R&D costs. At the same time, this increased revenue also represents increased costs to consumers, such as by delaying the market entry of presumably lower-cost generic products. TRIPS requires patent protection terms of a minimum of 20 years from the filing date. It does not require patent term extensions in cases of "unreasonable" delays by issuing authorities, but it does obligate members to ensure procedures, subject to conditions, for granting or registering patent rights within a reasonable period of time.99 Many FTAs include provisions for mandatory patent term length extensions beyond the TRIPS obligation of patent protection terms of twenty years from the filing date.
U.S. FTAs provide for extensions in cases of "unreasonable" delays in the issuance of patents due to regulatory review or administrative process that lessen the effective 20-year term of patent protection. NAFTA allowed countries to provide such an extension, but it did not define an unreasonable period of time. The U.S.-Chile FTA was the first U.S. FTA to define an unreasonable delay as one "to include a delay in the issuance of the patent of more than five years from the date of filing of the application in the Party, or three years after a request for examination of the application has been made (5-3 definition)."100 This level of protection was reprised in the Central American-Dominican Republic (DR-CAFTA). U.S. FTAs with Bahrain, Oman, and South Korea defined "unreasonable" as four years from the date of filing or two years after a request for examination.101 However, as a result of the May 10 Agreement, U.S. FTAs with Colombia, Panama, and Peru made patent term restorations in cases of unreasonable delays for pharmaceutical products optional, although it did contain the 5-3 definition of unreasonable in cases such obligations were undertaken. At the same time, these FTAs require the countries to make "best efforts to process patent applications and marketing approval applications expeditiously with a view to avoiding unreasonable delays."102
USMCA. In contrast, USMCA obliges each party to provide the means to a patent holder to adjust the term of a patent due to unreasonable delay, and requires each party to do so at the patent holder's request. USMCA returns to the earlier 5-3 definition.103
Patent Term Extension for Unreasonable Curtailment
An adjustment for unreasonable curtailment refers to adjusting for delays on account of the approval process for marketing new pharmaceutical products. Unlike most other products, manufacturers of pharmaceutical products cannot market them even after a patent is approved. The patent holder still needs to show the product is safe and effective to obtain marketing approval from a regulatory authority, such as the Food and Drug Administration (FDA) in the United States. This curtailment adjustment would ameliorate some of the time lost in obtaining marketing approval of the drug. This provision first appeared in the U.S.-Jordan FTA, and has continued in subsequent FTAs.104 It obligated parties to make available an extension of the patent to compensate for unreasonable curtailment without specifying a time period. For the Panama, Colombia, and Peru FTAs, this provision became optional.
USMCA. The USMCA renewed the obligation for parties to make available an extension of the patent to compensate for unreasonable curtailment. It also allows for the provision of a sui generis form of protection (e.g., a system not tied to the patent or marketing approval process). The POA, however, permits a party to attach conditions or limitations on this obligation. The amended text allows a party to restrict:
- the applicability of the article to a single extension;
- the adjustment to the first market approval granted;
- length of the extension to five years; and
- the length of additional sui generis protection to two years.105
Patent Linkage
Under the concept of patent linkage, if a patent currently is valid in a country, the pharmaceutical regulatory body of that country (i.e., the counterpart of the FDA) may not grant marketing approval for a generic version of that drug without the permission of the rights holder and must notify the rights holder if marketing is permitted. Patent linkage arguably strengthens patent protection, but may lengthen the time it takes for generic drugs to enter a market once the patent expires.
Neither TRIPS nor NAFTA contain patent linkage obligations. Without them, generic drug manufacturers could apply for marketing approval without the patent owner's permission and prior to the expiration of the patent. However, such generic manufacturers could still be sued for patent infringement. In contrast, patent linkage is a common requirement in many U.S. FTAs, beginning with the U.S.-Chile FTA.
Patent linkage provisions obligate the parties to:
- notify the patent holder of any third party requesting marketing approval effective during the term of the patent;106 and
- deny marketing approval to any third party prior to the expiration of the patent, except with the consent of the patent owner.107
The Colombia, Peru, and Panama FTAs reflected the policy changes of the May 10 Agreement, which sought to delink marketing approval from patent enforcement. The previous two principles became optional, and were joined, if applied, by the following obligations for a party to provide:
- "expeditious" administrative or judicial procedures to challenge the validity or applicability of a patent; and
- "effective" rewards to encourage the successful challenge to the validity or applicability of a patent.108
- USMCA. As originally negotiated, the patent linkage provision of the USMCA reverted back to the pre-May 10 standard, which prioritized enforcement of the patent and the ability to prevent generics from obtaining market approval prior to challenging the validity of the patent. Under the POA and in contrast to the May 10 FTAs, a party may provide for effective rewards for challenging the validity of a patent. A footnote to this provision suggests providing a period of marketing exclusivity to the first applicant that successfully asserts the invalidity or non-infringement of the patent as a potential reward. Also in contrast to the May 10 FTAs, a party may provide:
"Procedures…to promote transparency by providing information regarding applicable patents and relevant periods of exclusivity for pharmaceutical products that have been approved in that Party."
This language differs from the obligation to provide "expeditious administrative or judicial remedies" to challenge the validity of a patent per the language of the Colombia, Panama, and Peru agreements. 109
Protection of Test Data
Data exclusivity provides a period of protection for test data110 that prevents a generic company from relying on an innovator company's test data in order to gain marketing approval for a generic version of a brand name drug. During the data exclusivity period, the generic company would have to submit its own safety and effectiveness data with new drug trials to get regulatory approval. Since clinical trials and other testing data submitted for marketing approval can be costly and take years to develop, test data protection provides an incentive for innovation. At the same time, such provisions may delay access to generic forms of drugs. (See Figure 4.)
In cases in which the patent holders must submit undisclosed data regarding the safety or efficacy of new pharmaceutical or agricultural products (such as data from clinical trials) in order to obtain marketing approval, TRIPS requires members to take measures to protect such data from disclosure and unfair commercial use, and this requirement was reaffirmed in the U.S.-Jordan FTA.111 NAFTA further stipulated that such data could not be relied upon to support an application for marketing approval for a reasonable period of time, which was defined as "normally.…not less than five years" following the approval of the product by the producer of the data.112 For new chemical drug products, all subsequent U.S. FTAs, including USMCA, provided this minimum five-year period of data exclusivity, which typically begins from the date of marketing approval in the country. Beginning with the Singapore FTA, a party that provides a means of granting marketing approval based on the approval of the product in another country is required to defer approval for five years as well.113 The U.S.-Singapore FTA also began the inclusion in FTAs of a provision that would prevent data from being used for the full five years even if it outlasted the patent term.114
In addition, for the submission of new clinical information that includes a chemical entity previously approved for another pharmaceutical product (new uses for known products), the U.S.-Australia FTA began to require a minimum three-year period of data exclusivity for that data, which typically begins from the date of marketing approval in the country.115 Because the required availability of patent protection for new uses of a chemical entity previously approved for another use was removed in USMCA (see above), this companion period of data exclusivity was removed as well.
The Colombia, Panama, and Peru FTAs maintained five years of data exclusivity for test data related to new chemical products. However, they also included other provisions that may reduce the data exclusivity term by a minimum of six months in practice. If the FTA country relies on marketing approval granted by the FDA and grants approval within six months of an application for marketing approval by a person that produced the data, then the five-year period begins in the FTA country when the drug was first approved in the United States (oftentimes called the "concurrent period").116 As such, the data exclusivity period in the FTA country could run as long as the U.S. data exclusivity period, but no longer. The three-year data exclusivity period for previously approved chemical entities became optional.117
Regulatory Exclusivity for Biologics
Biological products ("biologics") are "large molecule" medical preparations derived from living organisms. Examples include vaccines, blood and blood components, and therapeutic proteins. Biologics are a relatively new area of pharmaceutical R&D.118 By contrast, "small molecules" chemical formulations traditionally have been the active substances in most pharmaceutical drugs.
Data exclusivity has a special significance for biologics. Since biologics are based on unique cell lines or biological processes, they cannot be replicated as generics as easily and inexpensively by relying on the originator product's efficacy and safety test data, as is the case for traditional small molecules-based medicine. Rather, regulatory agencies require more costly clinical trials to approve "biosimilars."119 U.S. law provides a 12-year period of data exclusivity for biologics.
Data exclusivity protection of biologics has been an increasing area of focus in trade negotiations. The United States first sought an additional period of exclusivity in the TPP negotiations, although other members were unwilling to accept the 12-year proposal from the United States.120 While the United States typically bases its proposals on existing U.S. law, it only sought a 10-year period of exclusivity in the USMCA. Although this level of protection was included in the USMCA as originally negotiated, the POA removed the exclusivity period for biologics entirely, a controversial decision that led innovator pharmaceutical groups to withdraw support from the agreement. 121 Canada currently provides a total of eight years of biologics exclusivity while Mexico provides a five-year exclusivity period for both small-molecule drugs and biologics.
Parallel Importation
Parallel imports, also known as grey-market goods, refer to goods imported into a country without permission of the rights holder after those goods were legitimately sold elsewhere. Parallel importation relates to the concept of territorial exhaustion of IPR, which governs the extent of IPR after the first sale. In many countries, IPR are exhausted at the first sale for any destination, and such goods can be exported or re-exported freely.122 Some developing countries contend that parallel importation is an alternative method for governments to increase access to medicines in the absence of a compulsory license.123 This practice also has implication for the importation of generic drugs into the United States. Pharmaceutical companies have voiced concerns that this practice threatens their ability to engage in price differentiation between different markets.
Article 6 of TRIPS specifically excludes issues arising from exhaustion of IPR from WTO dispute settlement, allowing each member to adopt different exhaustion regimes. Thus, TRIPS does not address the issue of parallel imports. U.S. FTAs negotiated with Australia, Singapore, and Morocco disallow parallel importing of patented products.124 Subsequent U.S. negotiated FTAs have not included this provision, due to language included in the Science, State, Justice, and Commerce, and Related Agencies, Appropriations Act of 2006 (P.L. 109-108), which prohibited the use of such provisions.
Copyright
In the area of copyright protection, the United States has pursued certain TRIPS-plus measures in FTAs, such as extending copyright terms, including anti-circumvention provisions, and protecting rights-management information in its FTAs. The TRIPS Agreement and NAFTA do not mention any obligations regarding technological protection measures or rights-management information, which is electronic information that identifies a protected work, its author, and terms and conditions of use,125 due to the fact these technologies were not available at the time. In contrast, U.S.-negotiated trade agreements prohibit the removal or alteration of such information.126
While patent protection has experienced policy shifts in the FTAs over the years, copyright protection provisions have remained fairly consistent. In general, U.S. FTA signatories are obligated to provide an additional twenty years of copyright protection from the TRIPS/NAFTA standard of 50 years after death of the author, bringing the minimum copyright term to seventy years from the death of the author. Responding to technological innovations not contained in the TRIPS Agreement, U.S. FTAs since the U.S.-Jordan FTA require trading partners to outlaw circumvention of "effective technological measures" to protect access to copyrighted works.127 USMCA was the first U.S. FTA specifically to distinguish technological protection measures128 from rights management information,129 while providing similar levels of protection for each. These provisions build on the U.S. Digital Millennium Copyright Act (DMCA) of 1998.130
Also based on the DMCA, U.S. FTAs since the U.S.-Chile FTA contain provisions that regulate the liability of Internet service providers (ISPs) for copyright infringement that occurs within their networks.131 Under the FTAs, ISPs are provided limited immunity from copyright liability in certain kinds of infringing situations if they comply with regulations known as notice-and takedown provisions. Under the notice-and-takedown process, ISPs must block access to or remove infringing materials as soon as they are made aware of the infringement by the rights holder, although ISPs users may file a counter-notice to restore material if they believe it is non-infringing. Copyright holders argue that it is necessary for ISPs to assist in enforcing copyright if copyright laws are to be effective in the online context. However, critics claim that these provisions impose excessive burdens on ISPs, reduce the rights of internet users, and limit the policy flexibility of FTA signatories in determining their own IPR regimes.
USMCA. For the most part, USMCA follows the standard copyright provisions found in U.S. FTAs noted above. Among the outcomes in the USMCA:
- Extension of copyright terms. NAFTA alone among U.S. FTAs contained the TRIPS 50-year standard. USMCA extends copyright terms from 50 years after death of the author (or 50 years from the publication) to 70 years after the death of the author (or publication). In addition, it increased the term of protection for works from other than a natural person (such as works made for hire) to 75 years from the year of the first authorized publication. Among the USMCA parties, only Canada maintained the 50-year term.
- Limitation and Exceptions. Confines "limitations and exceptions" to "certain special cases that do not conflict with the normal exploitation of the work…and do not unreasonably prejudice the legitimate interests of the rights holder." The USMCA does not contain additional language that was in the TPP to "endeavor to achieve an appropriate balance" between users and rights holders in their copyright systems, including digitally, through exceptions for legitimate purposes (e.g., criticism, comment, news reporting, teaching, research). The "appropriate balance" language speaks to fair-use exceptions in copyright law for media, research, and teaching. Rights-holder groups have criticized such provisions in the FTA context, while open internet groups have sought to have the fair-use provision inserted into the proposed USMCA.
- ISP "Safe harbor." Protects ISPs against liability for digital copyright infringement, provided that ISPs address intermediary copyright liability through "notice and takedown" or alternative systems (e.g., "notice and notice" in Canada). Rights-holder groups sought to limit what they considered "overly broad safe harbor provisions," while technology and business groups favored retention.
Trade Secrets
A company's ability to protect its commercially valuable proprietary information may affect its competitiveness or even its survival. Such proprietary information can include blueprints, chemical and other production processes, marketing strategies, or sales information. According to a 2014 survey by the ITC of more than 7,000 firms, 56% of internationally engaged firms considered trade secrets "very important."132
The USTR's 2019 Special 301 Report described the continued need for international protection and enforcement of U.S. trade secrets, citing the threat to U.S. competitiveness and risks to national security from the theft of U.S. trade secrets. The report highlights concern about inadequate protection and enforcement of trade secret law in certain countries. Companies are reportedly increasingly victimized by outright theft of their trade secrets, and have decried the often lax remedies available to combat such theft. Trade secret theft has taken on new and increased complexities in the digital environment, and the United States is increasingly concerned about trade secret theft through cybercrime. Penalties for trade secret theft vary widely among countries; some countries have no penalties at all while others have civil remedies or criminalize trade secret theft that results from computer hacking. In the United States, remedies for trade secret theft primarily are found in state law, although criminal and civil remedies are also available under federal law.
The USMCA is the first U.S. trade agreement since NAFTA to contain new provisions on trade secrets.133 NAFTA required each party to provide the legal means for a person to prevent trade secrets being disclosed without the consent of the person lawfully controlling the information.134 Subsequently, TRIPS language on "protection of undisclosed information" was derived from NAFTA.135 NAFTA also prohibited limiting the duration of trade secret protection or discourage or impede the voluntary licensing of trade secrets.
USMCA. In addition to the NAFTA language above, USMCA also requires each party to make available civil protection and criminal enforcement136 and penalties for unauthorized and willful misappropriation of trade secrets. However, it allows each party to determine the applicability of its procedures among at least one of the following:
- for the purposes of commercial advantage or financial gain;
- related to a product or service in national or international commerce; or
- intended to injure the owner of that trade secret.137
The other new feature of the USMCA trade secrets section is its prohibition on unauthorized disclosure of trade secrets by government officials in a legal or regulatory capacity outside the scope of their official duties.138
Trademarks
NAFTA defined trademarks as "any sign, or any combination of signs, capable of distinguishing the goods or services of one person from those of another, including personal names, designs, letters, numerals, colors, figurative elements, or the shape of goods or of their packaging."139 In addition, NAFTA defined trademarks to include service marks and collective marks (marks denoting organizations, such as associations, unions, or cooperatives) and may include certification marks (goods or services or providers have met certain standards.) With a few variances, recognition of collective and certification marks are required in U.S. FTAs.
The United States has used subsequent FTAs to include sound and scent marks in trademark protection. While NAFTA allowed parties to restrict trademarks to signs that are "visually perceptible," the U.S.-Singapore FTA and subsequent agreements prohibited countries from requiring marks to be visually perceptible.
The U.S.-Chile FTA was the first agreement to require trademarks for sound marks, and that requirement has been replicated in subsequent agreements.140 The United States has had less success in requiring scent marks. U.S. FTAs with Chile, Panama, and the DR-CAFTA countries provide that countries may include scent marks.141 More common is the language "neither party may deny registration solely on grounds of sounds and scents," which appears in the FTAs with Australia, Bahrain, Colombia, Oman, Peru, and South Korea.142 Singapore and USMCA require each party to make "best efforts" to register scent marks.143
U.S. FTAs generally, including the USMCA, provide for:
- a term of registration of no less than 10 years with the opportunity for 10-year periods of renewal indefinitely144 (whereas TRIPS requires a seven-year term and seven-year renewals);
- protection of well-known marks, whether registered or not, for goods and services for which they have gained their reputation; that protection may also be protected for dissimilar goods and services provided a connection exists with the goods and services of the owner;145
- the maintenance of a trademark classification system consistent with the Nice Agreement Concerning the International Classification of Goods and Services for the Purposes of the Registration of Marks;146
- limited exceptions such as fair use for descriptive terms; and
- appropriate measures to refuse an application or cancel a registration and prohibit the use of a trademark that is identical or similar to a well-known trademark by administrative procedures.
NAFTA contained several provisions that have not appeared in subsequent U.S. FTAs.147 However, these elements were incorporated into the TRIPS Agreement. They stipulated that:
- use of a trademark is not a prerequisite for filing an application for registration, although parties may make registration dependent on use;
- publication of each trademark must occur before registration or promptly after;
- parties shall require the use of a trademark to maintain a registration, and that a trademark may be cancelled after two years of non-use;
- an owner of a registered trademark may assign the trademark without the transfer of the business to which it belongs;
- parties shall recognize use of a trademark by a person other than the trademark owner, where such use is subject to the owner's control, as use of the trademark for purposes of maintaining the registration;
- compulsory licensing of trademarks is not allowed; and
- the nature of the goods or services to which a trademark is to be applied shall in no case form an obstacle to the registration of the trademark.
Internet Domain Names
NAFTA was negotiated before the widespread use of the internet, and does not contain language on internet governance. The U.S.-Chile and U.S.-Singapore FTAs were the first to contain language on domain names, which have been largely retained in subsequent U.S.-FTAs including USMCA.148 They require each party's country-code top-level domain (ccTLD) organization to:
- provide procedures to settle disputes based on principles established in the Internet Corporation of Assigned Names and Numbers' (ICANN) Uniform Domain-Name Dispute-Resolution Policy (UDRP),149 in order to address and resolve disputes related to the bad-faith registration of domain names in violation of trademarks; and
- provide a reliable and accurate database of contact information of domain name registrants.
Geographical Indications (GIs)
GIs are geographical names that protect the quality and reputation of a distinctive product from a specific region (e.g., Parma ham, Florida oranges). U.S. FTAs contain provisions on geographical indications in its IPR chapters, either freestanding or as part of the trademark section. For example, the trademark chapter requires that signs (e.g., brand logos) may serve as a geographical indication.
In FTA negotiations, the United States has sought to limit GI protections that, from the U.S. perspective, can improperly constrain U.S. agricultural market access in other countries by protecting terms it views as "common." USMCA defines a geographical indication as
an indication that identifies a good as originating in the territory of a Party, or a region or locality in that territory, where a given quality, reputation, or other characteristic of the good is essentially attributable to its geographical origin.150
Some previous agreements elaborated on the definition to include:
Any sign or combination of signs (such as words, including geographical and personal names, letters, numerals, figurative elements, and colors), in any form whatsoever, shall be eligible for protection or recognition as a geographical indication.151
GIs as Trademarks
Generally, U.S. FTAs have152 either required parties to recognize GIs as trademarks, or provide that parties may recognize GIs as trademarks or may be considered as certification marks eligible for trademark protection. NAFTA and the U.S.-Morocco FTA only required each party to provide owners remedies for GI infringement but they do not specifically refer to GIs' eligibility for trademark protection.153 USMCA requires only that "geographical indications may be protected through a trademark or a sui generis system or other legal means."154
Administrative Procedures
Most U.S. FTAs require parties to provide a means to apply or petition for protection or petition for recognition of a geographical indication and that the process adhere to certain norms and procedures. USMCA provides that if a party provides these administrative procedures, they must adhere to certain standards, which usually have appeared throughout U.S. FTAs. Parties shall:
- accept the application or petition without requiring intercession by a Party on behalf of its nationals;
- process those applications without imposing burdensome formalities;
- ensure the laws and regulation concerning GI application is readily available to the public; and
- provide contact information on the filing and administrative process concerning the application process and status of an application.155
More recent FTAs provide that these guidelines for administrative procedures outline the process for opposing applications or petitions as well. USMCA goes further by not only ensuring applications are published for opposition and procedures to oppose an application, but also to:
- provide a reasonable period of time for an interested person may oppose the application;
- require that administrative decisions in opposition proceedings be reasoned and in writing, which may be provided by electronic means;
- provide for cancellation of the protection or recognition afforded to a geographical indication; and
- require that administrative decisions in cancellation proceedings be reasoned and in writing, which may be provided by electronic means.156
Opposition, Denial, Cancellation
GI provisions in U.S. FTAs also include grounds for denial, opposition or cancellation. Earlier U.S. FTAs provided two specific justifications refusing protection:
- GI is confusingly similar to a preexisting pending good faith application for a trademark or a preexisting trademark registered in that Party; or
- GI is confusingly similar to a preexisting trademark, the rights to which have been acquired in accordance with the parties' law.157
The U.S.-Korea FTA (KORUS) added a third justification for refusing protection of a GI that is likely to cause confusion with a trademark that has become well known in the party's territory.158 USMCA replaces the additional KORUS justification to refuse protection for a term customary in common language as the common name for the relevant good in the territory of the Party.159 USMCA also sets out guidelines as to whether a term is the customary term for a good in common language.
USMCA is the first U.S. FTA to include applicable procedures if a party protects or recognizes a GI pursuant to an international agreement. This section may reflect the GIs recognized through FTAs Canada has signed with Canada and Mexico. The provisions largely track the notification, transparency, and opposition procedures above.
New and Evolving Issues
U.S. trade policy is increasingly focused on addressing new and evolving issues in international IPR protection and enforcement. The IPR landscape is changing, due to both the growing role of emerging markets in the global marketplace and the increased level of international trade taking place in the digital environment.
Indigenous Innovation
"Indigenous innovation" is a term and government industrial policy approach developed and deployed in China and other countries, including India. These policies generally aim to build out and advance China and other countries' science and technology, research and development, and industrial capabilities through discriminatory and protectionist policies. These policies commonly require foreign companies to localize operations in the country and force transfer of advanced proprietary IP and know-how to local domestic companies and government entities in an effort to develop "indigenous" capabilities. The policies also involve promoting innovation from domestic companies rather than relying on foreign technology, building domestic R&D capabilities more broadly, and increasing the share of overall value added by domestic companies to the domestic economy. Such innovation policies can surface in areas such as government procurement, technical standards, and technology transfer.160 For example, indigenous innovation policies may require the transfer of technology as a condition for allowing access to a market or for a company to continue to do business in the market.161 While the goal of increasing domestic manufacturing and innovation is acknowledged, the U.S. government, industry groups, and other stakeholders express concern that indigenous innovation policies are discriminatory and unfairly disadvantage U.S. right holders in those countries, and also may potentially violate WTO rules and disciplines. China's indigenous innovation policies, for example, have been a source of trade tension with the United States.
Localization Barriers to Trade
Functioning as a type of nontariff barrier to market access, "forced" localization measures generally refer to those designed to protect, favor, or stimulate domestic industries, service providers, or intellectual property at the expense of foreign counterparts. Localization barriers can take a number of forms, such as requirements for:
- service providers to process data in the foreign country as a condition of market access;
- businesses to transfer technology and intellectual property as a condition of approval of foreign investments; or
- firms to use local content as a condition for manufacturing or government procurement.
For example, with respect to India, U.S. businesses often cite localization requirements for data and servers as limiting market access and constraining innovation in the ICT sector.162 In China, transfer of technology policies require localization in a range of sectors such as medical equipment, electric vehicles, and information technology. While some localization barriers may serve data privacy or security objectives, concerns have arisen that some of these measures can be economically distorting. According to USTR, these measures can distort trade, inhibit FDI, and lead other countries to follow suit.163 Certain localization barriers have been addressed in multilateral trade negotiations. For instance, the WTO Agreement on Trade-Related Investment Measures (TRIMs) prohibits "local content" requirements imposed in a discriminatory manner with respect to foreign investment.164 Each of the above types of localization barriers are addressed in the USMCA, which prohibits forced technology transfer, data localization requirements, and local content rules.
Biodiversity and Traditional Knowledge
International trade negotiations increasingly have focused on the protection of inventions derived from plants and animals, new plant varieties, traditional knowledge, and folklore. Some indigenous communities in developing countries and international non-governmental organizations have expressed concern about the use of patents to provide private rights for traditional knowledge and genetic material. They also worry about the commercial use of such resources by entities other than the indigenous communities or countries from which such resources are derived, and the distribution of benefits from commercial use. The United States, other advanced countries, and business groups favor treating traditional knowledge and genetic material as eligible for intellectual property protection.
Article 27.3(b) of the TRIPS Agreement permits Member states to exempt "plants and animals other than micro-organisms, and essentially biological processes" from patentability. TRIPS requires Members to protect plant varieties through patent protection, a sui generis system, or a combination of the two. Paragraph 19 of the Doha Declaration added another dimension to the issue by requiring the TRIPS Council to probe the relationship between the TRIPS Agreement, the UN Convention on Biological Diversity (CBD), and traditional knowledge and folklore. These issues also are being discussed in WIPO's Intergovernmental Committee on Intellectual Property and Genetic Resources, Trade Knowledge, and Folklore.
Some earlier U.S. FTAs required signatories to provide protection for plants, animals, and plant varieties in their IPR chapters. FTAs with Peru, Panama, and Colombia do not mandate patentability for plants and animals, but state that the countries should take efforts to expand patent coverage to these areas and to maintain this protection once it is offered. Side-letters in these FTAs recognize the importance of biodiversity and traditional knowledge and pledge the countries to work together to address these issues. USMCA allows for parties to exclude animals and plants other than microorganisms from patentability, but that patents are available for inventions derived from plants.165 USMCA recognizes the importance of biodiversity in the Environment chapter.<del> </del>
Issues for Congress
Congress has legislative, oversight, and appropriations responsibilities related to IPR and trade policy. What follows are certain key issues that Congress could consider as it fulfills those responsibilities.
U.S. Efforts to Promote IPR Through Trade Policy
Since the inclusion of IPR provisions in NAFTA and the TRIPS Agreement, IPR protection and enforcement have been major U.S. trade policy negotiating objectives. Alongside the growing role of IPR in trade policy, there has been an ongoing debate regarding the appropriateness of linking IPR and trade policy. From one perspective, IPR could promote trade through innovation, economic growth, and technology transfer from advanced to developing countries. From another perspective, IPR, which grant legal temporary monopolies to rights holders for their creations, could be considered barriers to trade that have no place in trade liberalization negotiations. Given the continued use of trade policy to advance IPR objectives, debates also have focused on the appropriate balance between the protection and enforcement of IPR and other public policy objectives, such as access to medicines and the free flow of information, as well as the extent to which these goals are complementary or conflicting. Additionally, there have been debates about the trade policy channels used by the United States to promote IPR goals. Some question the appropriateness of using regional and bilateral FTAs for pursuing stronger IPR, contending that such actions take away from the effectiveness of multilateral IPR promotion efforts. Others argue that strong IPR commitments in U.S. regional and bilateral FTAs can provide momentum for developing such disciplines at the multilateral level. In light of the Administration's imposition of Section 301 tariffs on China and the limited treatment of IPR in the phase one deal struck between the United States and China, another issue Congress may consider is whether the use of Section 301 is an effective strategy to address IPR issues.
Another issue facing Congress is whether current U.S. trade negotiations comply with TPA objectives and congressional expectations. Congress may debate whether the results of the USMCA, particularly on pharmaceutical patents and exclusivity periods, are consistent with the TPA negotiating objective that agreements "reflect a standard of protection similar to that found in U.S. law," The alternative is that USMCA's IPR provisions represent a new paradigm for IPR chapters in future trade agreements, such as the newly announced negotiations for an FTA with Kenya. In addition, Congress may also consider whether partial trade liberalization, such as the agreement with Japan and negotiations with India and the European Union, sufficiently consider IPR issues.
Congress may use a possible renewal of TPA to reaffirm and or change U.S. trade negotiating objectives on IPR for future U.S. trade agreements. The current TPA expires on June 30, 2021. Congress may reassert current negotiating objectives to provide strong enforcement of IPR in trade agreements and to press for the elimination of government violations of IPR. It may also add new objectives or make adherence to existing objectives a criterion for initiating trade negotiations.
Enforcement of IPR Commitments
The extent to which U.S. FTA partners and WTO members are upholding their IPR commitments is of congressional concern. To date, the United States has concluded 14 FTAs with 20 countries. Some business observers argue that negotiating high-standard FTAs is not enough, and that "FTA commitments are meaningless if they are not consistently implemented and effectively enforced over time."166
Questions include whether existing U.S. trade policy tools, such as the "Special 301" process, bilateral consultations, and WTO and FTA dispute settlement mechanisms, are effective in bringing countries into IPR compliance. Aspects of these processes are subject to debate. For example, one question is whether "Special 301" designations are balanced in assessing countries' IPR regimes. Supporters contend that the Special 301 country designations—determined on a case-by-case basis and relying on interagency deliberations and consultations with Congress, foreign governments, and other stakeholders—accurately reflect countries' inadequacies in their IPR regimes. Others argue that the Special 301 is overly industry-driven and that country designations are not determined systematically.
Congress may examine whether there are additional opportunities for seeking redress for violations of TRIPS Agreement commitments through the WTO Dispute Settlement Mechanism. The United States has been a complainant in 18 (of 42) WTO disputes concerning the TRIPS Agreement, and has been a respondent in four more. Two U.S. cases have been filed since 2000—against China in 2007 and 2018 (described above). Congress may wish to consider the criteria by which USTR initiates cases, or evaluate the resources that may be necessary to investigate and bring additional cases. Some stakeholders also call for the United States to pursue greater trade enforcement action on IPR with respect to other countries.167
In addition, Congress could explore other options for advancing U.S. IPR trade policy objectives, including in the following areas:
- Bilateral Investment Treaties (BITs). Through the negotiation of BITs, the United States seeks to reduce barriers to foreign investment and strengthen protections for foreign investment.168 The U.S. Model BIT, the template the United States uses to negotiate BITs and investment chapters of FTAs, treats IPR as a covered form of investment subject to protections. Previously, the United States was negotiating BITs with China and India, but progress on these negotiations was constrained by differences in approaches. Congress could examine the progress of these negotiations, including how IPR issues are being addressed. Should these BIT negotiations be concluded, they would be subject to Senate ratification in order to enter into force.
- Tariffs. As noted above, Section 301 authorizes USTR to investigate and take action against U.S. trading partners that violate trade agreements or act in an "unjustifiable" or "unreasonable" manner to burden U.S. commerce. USTR has used this authority to counter China's forced technology transfer and IPR practices through the imposition of tariffs on Chinese goods. While Congress does not have a direct role in this process, it could recommend the use of Section 301 as a counter to IPR practices of other nations. Tariffs are one of many policy tools that can be deployed under Section 301. Congress could explore the other U.S. policy tools that have not yet been employed under Section 301 to address Chinese industrial policies and IP abuses–tools such as restrictions on Chinese investment and other commercial activities in the U.S. economy.
- U.S. trade preference programs. Some stakeholders point to U.S. trade promotion and preference programs as a potential tool for Congress to encourage policy reform in emerging economies. Should Congress take up GSP reauthorization, beneficiary countries may be subjected to heightened scrutiny over their IPR enforcement.
Effectiveness of the U.S. IPR Organizational Structure
A range of U.S. government agencies have responsibilities related to U.S. IPR activities. Some Members of Congress, private sector representatives, and other stakeholders express concern about whether the present U.S. IPR organizational structure is doing enough to enforce foreign countries' IPR obligations, as well as whether the structure is capable of doing more. (See Appendix A for more detail on U.S. agencies involved with IPR.)
One set of issues centers on coordination. Given the range of federal agencies involved in IPR protection and enforcement, questions have emerged about whether federal IPR activities are sufficiently coordinated in the present U.S. IPR organizational structure. On one hand, the Administration's establishment of various interagency bodies related to IPR, such as the Intellectual Property Enforcement Coordinator (IPEC), National Intellectual Property Rights Coordination Center (IPR Center), and ICTIME, affirms the U.S. commitment to enforcing IPR and the importance of interagency coordination. On the other hand, there are debates about whether the various IPR-related interagency coordinating mechanisms overlap. From one perspective, these interagency bodies focus on differing aspects of IPR protection and enforcement, and in doing so, collectively help to advance U.S. IPR goals in trade policy. From another perspective, the existence of multiple interagency coordinating bodies can contribute to additional bureaucracy.
Another set of issues centers on federal resources for IPR protection and enforcement. While protection and enforcement of IPR is a stated trade policy priority for the United States, it is difficult to get a sense of the magnitude of federal funding and resources devoted to it. Some U.S. government agencies do not have a separate budgetary line item for IPR-related activities, and Congress does not always designate specific funds for IPR activities in its appropriations for agencies. Additionally, information is limited on the economic and other impacts of piracy and counterfeiting on the United States. This may complicate the ability of lawmakers to weigh the threat of IPR infringement against the federal resources available for IPR and other government priorities. Furthermore, there could be debates about whether attempts to enhance interagency coordination, without devoting greater resources to IPR enforcement activities, may translate into greater U.S. IPR enforcement.
Looking Forward
U.S. efforts to protect and enforce IPR through U.S. trade policy are likely to continue to be of interest for Congress. The reliance on IPR as a competitive advantage to drive an innovative U.S. economy is reflected in U.S. trade policy. Congress may set the course of that policy concerning IPR through the development of negotiating objectives in any future trade promotion authority. It may evaluate the IPR provisions in the new USMCA as to whether they should become the template for future trade agreements. It may weigh the balance between greater intellectual property rights in free trade agreements and the ability to conclude agreements containing such provisions with other countries. It may examine how to incorporate the IPR aspects of new issues such as digital trade in U.S. policy.
Congress may also examine the enforcement of U.S. IPR through existing trade agreements, as well as the effectiveness of U.S. trade policy tools such as Special 301. Congressional debates may continue in areas such as how IPR protection and enforcement relate to other public policy goals, such as access to affordable medicines. The organizational structure for IPR protection and the priority to place on such enforcement when allocating budgetary resources also may be of congressional interest.
Appendix A. Overview of IPR-Related U.S. Government Agencies and Coordinating Bodies
What follows is a discussion of key U.S. government agencies and coordinating bodies involved in U.S. efforts to protect and enforce IPR.
Office of the United States Trade Representative (USTR)
The USTR is the lead U.S. trade negotiator and negotiates IPR provisions in U.S. trade agreements, at the multilateral, plurilateral, regional, and bilateral levels. It also enforces U.S. rights under existing trade agreements. Additionally, through its annual Special 301 Report, USTR is charged with monitoring the adequacy and effectiveness of IPR protection of the nation's trading partners, as well as their compliance with bilateral and multilateral trade agreements, identifying countries not in compliance with such agreements, and negotiating with those countries to improve compliance. The USTR further administers the Generalized System of Preferences (GSP) program, under which a country's eligibility for U.S. trade preferences may be contingent on its IPR protection.
Department of Commerce (Commerce)
Two agencies within the Department of Commerce, the Patent and Trademark Office and the International Trade Administration, address IPR issues.169
- The Patent and Trademark Office (PTO) administers the U.S. laws pertaining to patents and trademarks. It processes patent and trademark applications, issues patents and registers trademarks, considers petitions challenging patent validity, and conducts post grant reviews. The PTO develops IPR protection and enforcement policy and collaborates with other agencies to develop intellectual property provisions in FTAs and other international agreements. Additionally, the PTO offers training, technical assistance, and trade capacity building programs to assist in promoting strong IPR regimes in foreign countries through its IP Attaché Program.170 The PTO does not have jurisdiction over determining patent and trademark infringements; such determinations and remedies are made at the U.S. federal district court level or through the ITC's Section 337 proceedings. The PTO is fully funded through fees generated from patent and trademark applications.
- The International Trade Administration (ITA) administers many of the international trade programs of the Department of Commerce, including aspects involving IPR. The ITA monitors foreign countries' progress in implementing intellectual property agreements; reviews GSP petitions submitted by industry and coordinates the Commerce Department's response to these petitions; represents the Commerce Department at the WTO TRIPS Council; meets with trading partners to advance U.S. intellectual property interests abroad; and works with U.S. businesses and industry groups to make sure that IPR-related trade concerns are addressed.171
Department of Justice (DOJ)
The DOJ enforces criminal laws that protect IPR in the United States and internationally through the prosecution of intellectual property cases. Key units of the DOJ that have IPR enforcement responsibilities are the Criminal Division, U.S. Attorney's Office, the Civil Division, the Federal Bureau of Investigation, and the Office of Justice Programs.
- The Criminal Division prosecutes intellectual property crimes involving criminal offenses, namely through its Computer Crime and Intellectual Property Section (CCIPS).
- Federal prosecutors in the U.S. Attorneys' Offices pursue computer crime and intellectual property offenses.
- The Federal Bureau of Investigation (FBI) has an intellectual property enforcement program focusing on intellectual property crimes that have the most bearing on national and economic security, such as trade secret theft, internet priority, and counterfeit tracking goods. IPR is a top priority of the cyber division, though IPR crimes may be investigated in other divisions. Other IPR priorities for investigations are counterfeit health and safety products, and theft of trade secrets.
- The Civil Division prosecutes civil actions to recover penalties imposed by the Department of Homeland Security's Customs and Border Protection (CBP, discussed below) with respect to importation of counterfeit goods, brings affirmative cases when U.S. intellectual property rights are infringed, and defends CBP enforcement of the ITC's Section 337 exclusion orders, among other things.
- The Office of Justice Program awards grants to support intellectual property enforcement efforts by state and local law enforcement partners.
In addition to enforcement activities, the DOJ also works with Congress to develop laws that increase protection of IPR, and provides training and technical assistance programs on IPR enforcement through its Criminal Division.
Department of Homeland Security (DHS)
One of the aims of DHS is to ensure the facilitation of legitimate trade, while enforcing U.S. trade and IPR laws and investigating IPR violations, specifically trademark, counterfeiting, and copyright piracy. Key parts of DHS involved in IPR enforcement include U.S. Customs and Border Protection, U.S. Immigration and Customs Enforcement, U.S. Secret Service (USSS), and the National Intellectual Property Rights Coordination Center (IPR Center, discussed in next section).
- Customs and Border Protection (CBP) is responsible for detecting and seizing counterfeit and pirated goods entering the United States and determining penalties for infringement.172 CBP has the authority to determine whether or not imports infringe federally registered trademarks and copyrights and to detain or seize such infringing goods. Rights holders are able to record copyrights and trademarks with CBP's electronic IPR database and also notify the agency of possible IPR violations through an online reporting system.173 CBP cannot make determinations about patent infringements. However, it is able to block imports determined by the ITC to infringe a U.S. patent by a Section 337 investigation.174
- Immigration and Customs Enforcement (ICE) investigates violations of U.S. law that are connected with U.S. borders. ICE identifies, investigates, apprehends, and removes international criminal groups and other criminals. ICE conducts inquiries into the importation and distribution of counterfeit goods. ICE activities are closely linked with those of CBP. For instance, when CBP identifies and seizes counterfeit goods, the issue is referred to ICE for criminal investigation. Likewise, information obtained from ICE that is relevant to identifying and apprehending counterfeit shipments is provided to CBP.
- The U.S. Secret Service (USSS) investigates violations of laws relating to counterfeiting of obligations and securities of the United States; financial crimes; and computer-based attacks on U.S. financial, banking, telecommunications, and other critical infrastructure. As part of such activities, USSS may find links to IPR violations.
Department of Health and Human Services
The FDA, which is an agency of the Department of Health and Human Services (DHHS), is responsible for protecting public health by ensuring the safety and effectiveness of medicines, food, and other products. As part of its activities, the FDA works to protect consumers against counterfeit medicines. To combat the entry of foreign counterfeit drugs into the U.S. drug supply, the FDA works in conjunction with the CBP to conduct border inspections of FDA-regulated products. The FDA also engages in foreign inspections to ensure that foreign manufacturers meet FDA quality and labeling requirements. Funding to prevent counterfeits from entering the United States is part of overall FDA import safety efforts.175
Library of Congress
The Copyright Office of the Library of Congress administers U.S. copyright law by registering claims to copyright and related documents, including "assignments or transfers of rights" and maintains information on registrations, recordings, compulsory licenses, and other copyright-related actions. Additionally, the Copyright Office provides legal and technical expertise on national and international copyright issues to the U.S. government. The Copyright Office also works with other federal agencies to provide assistance and advice in negotiations for international intellectual property agreements, as well as technical assistance to foreign countries crafting their own copyright laws.176 Much like the PTO, the Copyright Office does not make copyright infringement determinations, which is generally the responsibility of federal district courts (or the ITC in Section 337 proceedings).
Department of State
The Department of State represents U.S. views in both bilateral and multilateral arenas. It works to build international consensus for IPR enforcement. Information from State's foreign postings informs the USTR Special 301 review. In particular, the Bureau of International Narcotics and Law Enforcement Affairs (INL) works to combat intellectual property piracy, while the Bureau of Economics and Business Affairs supports stronger international IPR standards to combat global piracy and counterfeiting.177
U.S. Agency for International Development (AID)
AID funds training and technical assistance to improve the compliance with the TRIPS Agreement and bilateral trade agreements with the United States. Funding for these projects generally have been undertaken by regional or country missions; there is no separate budgetary line item for IPR enforcement and training.178
United States International Trade Commission (ITC)
The ITC is a quasi-judicial federal government agency responsible for investigating and arbitrating complaints of unfair trade practices. It adjudicates allegations of imported products that infringe U.S. patents, trademarks, and copyrights through its Section 337 proceedings. The primary remedy employed by the ITC is to order the CBP to stop imports from entering the border. Additionally, the ITC may issue "cease and desist" orders against individuals determined to be IPR violators. Damages for IPR infringement cannot be received through ITC court proceedings; right holders seeking damages must file a civil action with a U.S. federal district court.179
Coordinating and Advisory Bodies
The USTR leads interagency coordination of U.S. trade policy formulation, negotiation, and implementation. Beyond this general mechanism, the U.S. government also has interagency coordination for IPR protection and enforcement activities, as well as private sector advisory bodies that provide input into the formulation of U.S. trade policy. Certain key coordinating and advisory bodies are outlined below.
Office of the U.S. Intellectual Property Enforcement Coordinator (IPEC)
The IPEC, located in the Office of Management and Budget (OMB) of the Executive Office of the President, provides executive direction and coordination of federal agencies involved in IPR enforcement. The position of the U.S. Intellectual Property Enforcement Coordinator, subject to Senate confirmation, was statutorily established in October 2008, through the Prioritizing Resources and Organization for Intellectual Property Act of 2008 (P.L. 110-403).180 Among its key responsibilities are to develop and implement a "Joint Strategic Plan on Intellectual Property Enforcement" for combating counterfeiting and piracy; provide assistance to the USTR in conducting trade negotiations relating to IPR enforcement abroad; and chair an Advisory Committee composed of representatives from the OMB; Departments of Justice, Commerce, State, Homeland Security, and Agriculture; FDA; AID; and the Register of Copyrights.
FY2017-FY2019 Joint Strategic Plan on Intellectual Property Enforcement
|
The U.S. Intellectual Property Enforcement Coordinator (IPEC), assisted by its Advisory Committee, is charged with developing a "Joint Strategic Plan" for combating counterfeiting and piracy. FY2017-FY2019 Joint Strategic Plan on Intellectual Property Enforcement. In December 2016, IPEC released its third Joint Strategic Plan on Intellectual Property Enforcement for FY2017-2019, which noted progress and areas for future activity in major areas of focus: (1) enhance IPR enforcement in other countries; (2) promote IPR enforcement in U.S. trade agreements; (3) enhance domestic and international patent protection; (4) recognition of the role of universities in innovation; (5) mitigate the theft of U.S. trade secrets; and (6) promote supply chain accountability in government acquisition. The Trump Administration sought comments for a new 3-year Joint Strategic Plan in the Federal Register on September 13, 2018. |
Source: Executive Office of the President, FY2017-2019 Joint Strategic Plan on Intellectual Property Enforcement, December, 2016, https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/IPEC/2016jointstrategicplan.pdf.
National Intellectual Property Rights Coordination Center (IPR Center)
The Department of Homeland Security houses the IPR Center, an interagency task force whose mission is "to ensure national security by protecting the public's health and safety, the U.S. economy, and our war fighters, and to stop predatory and unfair trade practices that threaten the global economy." Established by ICE in 2002, the IPR Center's role is to improve and coordinate federal intellectual property functions to more effectively combat IPR-infringing products. It is led by the ICE Homeland Security Investigations (HSI) Director, with Deputy Directors from HSI and CBP. According to USTR, the IPR Center can be distinguished from ITEC (discussed below) because of the former's focus on the law enforcement response to IPR theft (primarily coordinating investigation and prosecution of IPR infringers under U.S. criminal laws) and the latter's focus on enforcement of U.S. rights under trade agreements across a range of issues, one of which is IPR.181
Interagency Center for Trade Implementation, Monitoring, and Enforcement (ICTIME)
ICTIME succeeds the Interagency Trade Enforcement Center (ITEC), which was established February 28, 2012, by Executive Order.182 ICTIME was established under section 604 of the Trade Facilitation and Trade Enforcement Act of 2015 (P.L. 114-125). The center is primarily staffed by USTR employees and its director is appointed by the USTR; other federal agencies may detail employees to the center.183 Its purpose is to advance U.S. trade policy through strengthened and coordinated enforcement of U.S. trade agreements, including IPR. ICTIME investigates potential disputes under the auspices of the World Trade Organization; inspects potential disputes pursuant to bilateral and regional trade agreements to which the United States is a party; and carries out the functions of USTR with respect to the monitoring and enforcement of trade agreements to which the United States is a party. USTR and ITA work closely within the ICTIME to identify issues and develop information in areas of economic importance to U.S. industries. In 2019, ICTIME supported the Section 301 investigation on China regarding intellectual property and technology transfer.184
Private Sector Advisory Committee System
The USTR manages a private sector advisory committee system for trade policy, intended to provide information and advisory on U.S. negotiating objectives and bargaining positions before the United States enters into trade agreements, the operation of existing U.S. trade agreements, and other U.S. trade policy matters.185 Statutorily established under section 135 of the Trade Act of 1974 (P.L. 93-618), the private sector advisory system includes 16 Industry Trade Advisory Committees (ITACs), which are jointly administered by the USTR and Department of Commerce. ITAC membership draws from industry and labor; one of the ITACs focuses on IPR.186
|
COVID-19 and Access to Medicine The Coronavirus Disease 2019 (COVID-19) pandemic may reopen a debate over the relationship between WTO trading rules and countries' ability to obtain needed drugs or vaccinations. As noted above, TRIPS created the first enforceable minimum standards for international IPR. It affirmed that patents "shall be available for any inventions…in all fields of technology, provided that they are new, involve an inventive step and are capable of industrial application." It also applied the principle of nondiscrimination on issuance of patents based on technology, place of invention, or site of use. This standard was particularly important to innovative pharmaceutical manufacturers because several countries did not provide for patenting pharmaceutical products prior to TRIPS, or, as in the case of India, provided process patents that covered the manufacturing process but not product itself. However, TRIPS does provide for limited exceptions to the patent right. For example, a country may limit patent rights provided the limitation does not "unreasonably" conflict with the normal exploitation of a patent. The agreement also contains exceptions allowing a party to exclude from patentability items to protect human life and health, as well as diagnostic, therapeutic and surgical measures. The Doha Declaration on TRIPS and Public Health (see above) affirmed that TRIPS provisions should be interpreted to promote public health and access to medicine. TRIPS also allows for compulsory licensing, but places limitations on its use. A compulsory license is an authorization by a government for third parties (such as a company or the government itself) to manufacture or use a product under patent without the permission of the rights holder. TRIPS permits signatories to issue compulsory licenses for patented inventions, if the third party attempts to obtain permission from the patent holder and negotiates reasonable commercial terms, although this requirement can be waived in times of national emergency or other extenuating circumstances. In any case, the third party must provide "adequate" remuneration to the patent holder for the use of the patent. Another restriction limits its use primarily to the domestic market, although countries may issue compulsory license to send products to least-developed countries that lack domestic production capabilities. The allowance for least-developed countries and a clarification of the meaning of national emergency became part of the amendment to TRIPS that originated with the Doha Declaration. U.S. bilateral and regional FTAs largely have not addressed the issue of compulsory licensing, but have contained provisions incorporating the Doha Declaration. In practice, the use of compulsory licenses has been rare; the threat of invoking a compulsory license as a negotiating tactic for countries to obtain better prices from a manufacturer has been more common. The United States generally has sought to dissuade other nations from using compulsory licensing, even placing greater limitations on its use in early U.S. FTAs with Australia, Singapore, and Jordan. However, with the COVID-19 virus, it has been reported that certain governments are taking preliminary steps to revisit its use. Israel is the first country to issue a compulsory license in the context of COVID-19 for the AbbVie drug Kaletra (lopinavir/ ritonavir). The next day AbbVie announced it would no longer enforce patents worldwide for lopinavir/ritonavir.56 In March 2020, the parliaments of Canada and Germany passed legislation clarifying or streamlining the ability to use compulsory licenses in their countries. The National Assemblies of Chile and Ecuador are calling for the use of compulsory licenses in fighting the COVID-19 pandemic.57 For more information see, see CRS Legal Sidebar LSB10436, COVID-19: International Trade and Access to Pharmaceutical Products, by Nina M. Hart. |