Early Development and Regulation of Diagnostic Testing for COVID-19: Frequently Asked Questions
Changes from March 9, 2020 to March 17, 2021
This page shows textual changes in the document between the two versions indicated in the dates above. Textual matter removed in the later version is indicated with red strikethrough and textual matter added in the later version is indicated with blue.
Early Development and Regulation of Domestic Diagnostic Testing for Novel Coronavirus (COVID-19): Frequently Asked Questions
Contents
- Diagnostic Tests
- What Are IVD Tests?
- What Is an LDT?
- How Are IVD Tests Regulated?
- How Are LDTs regulated?
- What Is CLIA and How Is It Involved in LDT Regulation?
- How Are IVDs Regulated by the FDA During an Emergency Such as the Outbreak of COVID-19?
- How Does the Emergency Use Authority Apply to LDTs if They Are Generally Exempted from Premarket Requirements?
- The CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time Reverse Transcriptase (RT)-PCR Diagnostic Panel
- How Does the CDC's COVID-19 Diagnostic Test Work?
- What Type of IVD Is the CDC's Test and Who May Carry It Out?
- What Is the Role of the Commercial Manufacturer IDT in the Development and Distribution of the CDC's Test Kit?
- What Quality Problems Did the CDC's Test Experience on Rollout to the State and Local Public Health Laboratories?
- What Steps Did FDA Take to Expand Testing Capacity in Response to the Problems with CDC's Test?
Summary
17, 2021
Diagnostic Testing for COVID-19: Frequently
Amanda K. Sarata
Asked Questions
Specialist in Health Policy
On December 31, 2019, the World Health Organization (WHO) was informed of a cluster of pneumonia cases in Wuhan City, Hubei Province of China. Illnesses have Il nesses have
since been linked to a disease caused by a previously unidentified strain of coronavirus,
designated Coronavirus Disease 2019, or COVID-19. Despite containment efforts in China, the United States, and elsewhere, by late February there were indications that the COVID-19 outbreak may havehad entered a new phase, with community spread occurring or suspected in several countries other than China, including in the United States.
Since this time, the virus has spread widely, resulting in mil ions of cases and more than 500,000 deaths in the United States.
Diagnostic testing is a critical part of the public health response to and clinical management of COVID-19, the
disease caused by the SARS-CoV-2 virus. EffortsThe earliest efforts in the United States to develop and disseminate a test for COVID-19 have faced challengesfaced chal enges. Manufacturing and quality issues with the nation's ’s first test—developed by the Centers for Disease Control and Prevention (CDC)—resulted in significant delay indelayed access to testing throughout the country. In this context, on February 29, 2020, in an effort to facilitate the expansion of testing capacity as the first cases of community spread were confirmed in the United States, the Food and Drug Administration (FDA) announced a
new COVID-19 diagnostics policy. The new policy, issued via agency guidance and effective immediately, that would allow certain laboratories—principally clinical and large commercial and reference laboratories—that haveal owed certain laboratories that had developed and validated their own COVID-19 diagnostic to begin to use the test prior to it receiving an Emergency Use Authorization (EUA) from the agency.
FDA'
FDA’s February 29 guidance aims to facilitate the (subsequently updated March 16, May 4, and May 11 of 2020) supported the expansion of diagnostic testing from the public health setting into the clinical health care and commercial settings. Doing so may
An expansion of diagnostic testing was part of an early effort to help the country meet the increasing and substantial demand for testing. Increasing demand was generated primarily generated by community spread of the disease and expanded clinical testing guidelines issued by the CDC. This report does not address financing or coverage of diagnostic testing for COVID-19.
O at the time.
Congressional Research Service
link to page 6 link to page 6 link to page 6 link to page 7 link to page 7 link to page 8 link to page 8 link to page 8 link to page 9 link to page 9 link to page 10 link to page 10 link to page 10 link to page 10 link to page 11 link to page 11 link to page 12 link to page 12 link to page 13 Early Development and Regulation of Diagnostic Testing for COVID-19
Contents
Diagnostic Tests.............................................................................................................. 3
Which Federal Agencies Have a Role in Diagnostic Test Regulation? ................................ 3
What Are IVD Tests? ................................................................................................. 3 How Are IVD Tests Regulated? ................................................................................... 4
How Are LDTs Regulated? .................................................................................... 4 What Is CLIA and How Is It Involved in LDT Regulation? ......................................... 5
How Are IVDs Regulated by the FDA During an Emergency Such as the COVID-19
Pandemic? ............................................................................................................. 5
How Does the Emergency Use Authority Apply to LDTs If They Are General y
Exempt from Premarket Requirements? ................................................................ 6
The CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time Reverse Transcriptase (RT)-
PCR Diagnostic Panel ................................................................................................... 7
How Does the CDC’s COVID-19 Diagnostic Test Work? ................................................ 7 What Type of IVD Is the CDC’s Test and Who May Carry It Out? .................................... 7 What Quality Problems Did the CDC’s Test Experience on Rollout to the State and
Local Public Health Laboratories? ............................................................................. 8
What Steps Did FDA Take to Expand Testing Capacity in Response to the Issues with
CDC’s Test? ........................................................................................................... 9
Contacts Author Information ....................................................................................................... 10
Congressional Research Service
Early Development and Regulation of Diagnostic Testing for COVID-19
n December 31, 2019, the World Health Organization (WHO) was informed of a cluster of pneumonia cases in Wuhan City, Hubei Province of China. IllnessesIl nesses have since been
O linked to a disease caused by a previously unidentified strain of coronavirus, designated
Coronavirus Disease 2019, or COVID-19. Despite containment efforts in China, the United States, and elsewhere, by late February there were indications that the COVID-19 outbreak may have entered a new phase, with community spread occurring or suspected in several countries
other than China, including in the United States.1
Since this time, the virus has spread widely,
resulting in mil ions of cases and more than 500,000 deaths in the United States.
Diagnostic testing is a critical part of the public health response to and clinical management of the COVID-19 caused by the SARS-CoV-2 virus. EffortsCOVID-19. The earliest efforts in the United States to develop and disseminate a test for COVID-19 faced chal enges19 have faced challenges. Manufacturing and quality issues with the nation'’s test—developed by the Centers for Disease Control and Prevention (CDC)—resulted in essentially all essential y al testing going through CDC'’s laboratory facility in Atlanta through early March 2020, despite distribution of test kits to state and local public health labslaboratories beginning in early February 2020. CDC's initial test kits’s
initial test kit had to be remanufactured and redistributed, which, along with other factors, has significantly delayed access to testing throughout the country. It has been reported that the CDC's Atlanta laboratory is currently early in the pandemic.1 Early reports indicated that the CDC’s Atlanta laboratory was under investigation by the Department of Health and Human Services (HHS) for possible quality issues related to its manufacture of the test kits, which may have led to the contamination of one reagent and thus to the quality issues with the test.2
test.2 In June 2020, HHS Office of the General Counsel released findings of an internal investigation into CDC’s production of its test kit, finding general y that the test was likely contaminated, and that time pressure may have “compromised sufficient QC/QA [quality control/quality assurance] to identify certain anomalies in data and realize the possibility of contamination before shipment.”3 In addition, the matter is currently under investigation by HHS
Office of the Inspector General, with an audit underway to “review CDC’s process of producing and distributing the COVID-19 test kits.”4 This report is reportedly expected sometime in
FY2021.
In this context, on February 29, 2020, in an effort to facilitate the expansion of testing capacity as the first cases of community spread were confirmed in the United States, the Food and Drug Administration (FDA) announced a significant newnew COVID-19 diagnostic testing policy. This policy, issued via agency guidance and effective immediately, allowsal owed certain laboratories—principallyprincipal y clinical and commercial laboratories—that havehad developed and validated their own COVID-19 diagnostics
to begin to use the tests prior to the test receiving an Emergency Use Authorization (EUA) from the agency.3
Diagnostic testing for COVID-19, in part because it is caused by a novel pathogen, has been led and carried out to date through the country's public health infrastructure. This includes primarily the CDC and the country's network of state and local public health laboratories. In contrast, in most situations, diagnostic testing is carried out by a number of facilities, including private commercial laboratories (e.g., Quest, LabCorp), hospital and other clinical laboratories, and laboratories in academic medical centers, among others.
FDA's February 29 guidance aims to facilitate5 According to reporting at the time, this meant “the nation wil become able virtual y 1 University of Minnesota Center for Infectious Disease and Policy, “Glitch delays COVID-19 tests for states as first evacuees cleared,” February 12, 2021, https://www.cidrap.umn.edu/news-perspective/2020/02/glitch-delays-covid-19-tests-states-first-evacuees-cleared.
2 “U.S. agency investigating production of faulty coronavirus test kits,” March 1, 2020, https://www.reuters.com/article/us-china-health-test-kits/u-s-agency-investigating-production-of-faulty-coronavirus-test-kits-idUSKBN20P09Y. 3 Washington Post, “Summary of the Findings of the Immediate Office of the General Counsel’s Investigation Regarding CDC’s Production of COVID-19 T est Kits,” https://www.washingtonpost.com/context/summary-of-the-findings-of-the-immediate-office-of-the-general-counsel-s-investigation-regarding-cdc-s-production-of-covid-19-test-kits/a750fbf7-9a4f-4062-8fcc-c6cf42600578/.
4 HHS OIG, “Audit of HHS’s Production and Distribution of COVID-19 Lab T est Kits,” https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000462.asp.
5 See generally FDA, “Policy for Diagnostics T esting in Laboratories Certified to Perform High Complexity T esting under CLIA prior to Emergency Use Authorization for Coronavirus Disease -2019 during the Public Health Emergency,” May 11, 2020, https://www.fda.gov/media/135659/download; and FDA, “Coronavirus (COVID-19) Update: FDA Issues New Policy to Help Expedite Availability of Diagnostics,” https://www.fda.gov/news-events/
Congressional Research Service
1
Early Development and Regulation of Diagnostic Testing for COVID-19
overnight to test thousands of patients rather than the few hundred tested so far for the virus, known as Covid-19.”6 This guidance, updated March 16, May 4, and May 11 of 2020, has been a
pivotal part of FDA’s response to diagnostic testing during the pandemic.
In part because COVID-19 is caused by a novel pathogen, diagnostic testing was initial y led and carried out through the country’s public health infrastructure. This infrastructure includes the CDC and the country’s network of state and local public health laboratories. In contrast, in normal situations with established pathogens, diagnostic testing is carried out by a number of entities, including private reference and commercial laboratories (e.g., Quest, LabCorp), hospital-
based and other clinical laboratories, and laboratories in academic medical centers, among others. During the pandemic, community-based testing sites, drive-through testing sites, and retail pharmacies al have played a role in providing COVID-19 testing, both in terms of sample
collection as wel as providing point-of-care testing.
FDA’s COVID-19 diagnostics guidance supported the expansion of diagnostic testing from the public health setting into the clinical health care and commercial settings, leveraging significant standing resources throughoutacross the country, including facilities, trained personnel, expertise, materials, and equipment. It iswas FDA'’s intention that this expansion willof diagnostic testing would help the
help the country meet the increasing and substantial demand for testing generated both by community spread of the disease and COVID-19 as wel as expanded clinical testing guidelines issued by the CDC.CDC at the time.7 In addition, because many cases of COVID-19 are reportedly mild or asymptomatic, widespread access to testing—which informs development of important metrics such as the case fatality rate—iswas critical to understanding the scope and extent of the disease in the United States, and to efficiently directing resources to
mitigate its impact in the broader community.
Diagnostic tests—formally called, including in schools and workplaces.
Diagnostic tests—formal y cal ed in vitro diagnostic (IVD) devices—may be commercially commercial y developed and distributed as "kits"“kits” or developed, validated, and carried out by a single clinical
laboratory. This second type of test—known as a laboratory-developed test, or an LDT—is, when carried out in a single laboratory, is referred to by FDA as a laboratory-developed test (LDT), and is typical y the more commonly used type of test in rapidly evolving situations because of its flexibility and differing federal regulatory requirements, among other reasons. Although the CDC'CDC’s test is being manufactured as a test kit and initially has been distributed to specific CDC-qualified labs, the FDA guidance targets LDTs that are generally carried out in clinical and academic settings or private commercial laboratories. All clinical laboratories in the United States, regardless of whether they are part of the country's public health infrastructure or part of the health care delivery system, are regulated by the Clinical Laboratory Improvement Amendments of 1988 (CLIA) program, administered by the Centers for Medicare & Medicaid Services (CMS).
Federal agencies involved in the regulation of IVDs include FDA and CMSwas manufactured as a test kit and initial y was authorized to be distributed only to specific CDC-qualified labs. FDA’s diagnostics guidance eventual y applied to both manufacturers of “kits” as wel as to clinical laboratories carrying out tests they developed
and validated. The initial February 29 version of the guidance focused on tests carried out by high-complexity clinical laboratories only, including LDTs, as these tests were in many cases already developed and validated, and were able to be offered almost immediately, without needing to be manufactured and commercial y distributed as kits. Two of the country’s largest clinical laboratories, Quest Diagnostics and LabCorp, almost immediately began carrying out
their own COVID-19 molecular diagnostic testing in early March pursuant to the FDA guidance.8
press-announcements/coronavirus-covid-19-update-fda-issues-new-policy-help-expedite-availability-diagnostics.
6 Wall Street Journal, February 29, 2020, “FDA to Allow Labs to Begin Use of High -Complexity T ests for Coronavirus,” https://www.wsj.com/articles/fda-to-allow-labs-to-begin-use-of-high-complexity-tests-for-coronavirus-11582992472?mod=article_inline.
7 FDA, “Coronavirus (COVID-19) Update: FDA Issues New Policy to Help Expedite Availability of Diagnostics,” https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-new-policy-help-expedite-availability-diagnostics.
8 See for example, Quest, “COVID-19: Doing our Part,” https://www.questdiagnostics.com/home/Covid-19/#:~:text=March%209%202020,was%20labeled%20a%20worldwide%20pandemic.
Congressional Research Service
2
Early Development and Regulation of Diagnostic Testing for COVID-19
Diagnostic Tests
Which Federal Agencies Have a Role in Diagnostic Test Regulation? Federal agencies involved in the regulation of IVDs include FDA and the Centers for Medicare &
Medicaid Services (CMS). FDA derives its authority to regulate the sale and distribution of medical devices, such as IVDs, from the Federal Food, Drug, and CosmeticsCosmetic Act (FFDCA) and the Public Health Service Act (PHSA). CMS'’s authority to regulate IVDs is through CLIA (P.L. 100-578). the Clinical Laboratory Improvement Amendments of 1988 (CLIA, P.L. 100-578), codified in the PHSA.9 FDA regulates the safety and effectiveness of diagnostic tests, as well wel as the quality of the design
and manufacture of the diagnostic test.10 CMS regulates the quality of clinical laboratories and the clinical testing process.
Diagnostic Tests
11 Al clinical laboratories in the United States, regardless of whether they are part of the country’s public health infrastructure or part of the health care delivery system, are regulated by the CLIA program, administered by CMS. The CLIA program general y certifies clinical laboratories as able to carry out high or moderate complexity tests, or waived tests
(usual y referred to as point-of-care tests).
What Are IVD Tests? What Are IVD Tests?
In vitro diagnostic devices are defined in FDA regulation as a specific subset of medical devices that include "“reagents, instruments, and systems intended for use in the diagnosis of disease or other conditions ... in order to cure, mitigate, treat, or prevent disease ... [s]uch products are intended for use in the collection, preparation, and examination of specimens taken from the human body."”12 As indicated by this definition, an IVD may also include componentscomponents of tests,
which can include both non-diagnostic ingredients, calledcal ed general purpose reagents (GPRs), and
the active ingredient(s) in a diagnostic test, referred to as the analyte specific reagent (ASR).
In general, as noted above, an IVD device may be a "commercial test kit" (a (a self-contained
commercial product developed, produced, and solddistributed by a manufacturer for distribution to multiple laboratories) or a "laboratory developed test" (a product developed by and used in a single clinical laboratory). LDTs may use components (e.g., general purpose reagents like a buffer) that are either manufactured in-house by the laboratory or commercially developed and distributed.
What Is an LDT?
A laboratory-developed test isacquired by the laboratory commercial y, and
were previously referred to as “home-brew tests.”
The FDA defines an LDT as a class of IVD that is designed, manufactured, and used within a singlesingle clinical laboratory.13 LDTs are often used to test for conditions or diseases that are either rapidly changing (e.g., new strains of known infectious diseases) or are the subject of quickly advancing
scientific research (e.g., genomic testing for cancer). The majority of genetic tests—a type of IVD that analyzes various aspects of an individual'’s genetic material (e.g., DNA, RNA)—are LDTs.
9 PHSA §353 [21 U.S.C. §263a]. 10 FDA, “Overview of IVD Regulation,”https://www.fda.gov/medical-devices/ivd-regulatory-assistance/overview-ivd-regulation. 11 CMS, “Clinical Laboratory Improvement Amendments (CLIA),” https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA.
12 21 C.F.R. §809.3(a) 13 FDA, “Framework for Regulatory Oversight of Laboratory Developed T ests (LDT s),” October 3, 2014, https://www.fda.gov/media/89841/download.
Congressional Research Service
3
Early Development and Regulation of Diagnostic Testing for COVID-19
Many COVID-19 diagnostics are LDTs, most commonly molecular tests such as polymerase
chain reaction (PCR) tests.
How Are IVD Tests Regulated? s genetic material (e.g., DNA, RNA)—are LDTs.
How Are IVD Tests Regulated?
In general, oversight of in vitro diagnostic devices focuses on ensuring their safety and effectiveness; their accuracy and reliability; the quality of clinical laboratories that carry out IVD testing; the utility of the information generated by IVDs in clinician and patient decision-making; and the truthfulness of claims made about IVDs that are marketed directly to consumers. As with other medical devices, the application of FDA regulatory requirements to IVDs depends on the IVD'’s risk classification according to its intended use. Classification is based, in turn, on the risk the device poses to the patient.14 For IVDs, which are informational tests, the risk to the patient is that of an incorrect test result, either a false positive or a false negative result, either of
which may cause serious harm to the individual. In the case of infectious diseases—for example, COVID-19—the risk of a false negative test extends beyond the individual patient into the community at large. The FDA has three classes of medical deviceIn addition, false positive results may result in wasted clinical resources and exposure of healthy individuals to infected individuals. The FDA classifies medical devices based on their risk to the consumer: Class I (low risk), Class II (moderate risk), and Class III (high risk). Regulatory controls are dependent on the class of a given medical device.15 If the manufacturer
was seeking to market a test outside of an emergency situation, a COVID-19 diagnostic would likely fal into Class II, requiring clearance (through 510(k) notification) prior to marketing, or possibly Class III, requiring a premarket approval (PMA) prior to marketing. In the case of a novel low- or moderate-risk product, it could receive marketing authorization through FDA’s De
Novo pathway.16
COVID-19 diagnostics would most likely fall in Class III, as they would be considered to be high-risk devices.
How Are LDTs Regulated?
How Are LDTs Regulated?
The regulation of laboratory-developed testsLDTs has been the subject of ongoing debate over at least the past 20 years, driven in large part by an increase in the number and complexity of genetic tests over this time. 17 In general, the FDA has maintained that it has clear regulatory authority over LDTs, as it does with all al IVDs that meet the definition of medical device in the FFDCA.418 However, the FDA traditionally
traditional y exercised enforcement discretion over LDTs—choosing not to enforce applicable statutory and regulatory requirements with respect to such tests—meaning that most of these tests have neither undergone premarket review nor received FDA clearance or approval for marketing.19 To date, FDA has instead focused its enforcement efforts on commercial IVDtest kits, which are broadly commerciallycommercial y marketed. In recent years, despite the absence of specific agency guidance on the
regulation of LDTs, FDA has nevertheless begun to assert authority over LDTs, and specifically over some direct-to-consumer (DTC) genetic tests, that it considers to be higher-risk tests.
certain LDTs that it
considers to be higher-risk.20
14 FDA, “Overview of IVD Regulation,” https://www.fda.gov/medical-devices/ivd-regulatory-assistance/overview-ivd-regulation.
15 See CRS In Focus IF11083, Medical Product Regulation: Drugs, Biologics, and Devices. 16 See FDA, “FDA Permits Marketing of First SARS-CoV-2 Diagnostic T est Using T raditional Premarket Review Process,” https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-first-sars-cov-2-diagnostic-test-using-traditional-premarket-review-process.
17 See CRS In Focus IF11389, FDA Regulation of Laboratory-Developed Tests (LDTs). 18 T he term device is defined in FFDCA §201(h) [21 U.S.C. §321(h)]. 19 FDA, “Laboratory Developed T ests,” https://www.fda.gov/medical-devices/vitro-diagnostics/laboratory-developed-tests.
20 FDA Law Blog, Hyman, Phelps, and McNamara, “Regulation of Laboratory Developed T ests by FDA: T ime for the Agency to Cease and Desist Until Congress Enacts Legislation,” https://www.fdalawblog.net/2019/10/regulation-of-laboratory-developed-tests-by-fda-time-for-the-agency-to-cease-and-desist-until-congress-enacts-legislation/.
Congressional Research Service
4
Early Development and Regulation of Diagnostic Testing for COVID-19
What Is CLIA and How Is It Involved in LDT Regulation?
The Clinical Laboratory Improvement Amendments of 1988 (CLIA) providedWhat Is CLIA and How Is It Involved in LDT Regulation?
CLIA of 1988 provides CMS with authority to regulate clinical laboratories.521 CLIA establishes quality standards for clinical laboratory testing and a certification program for clinical laboratories that perform testing using IVD devices. All laboratoriesAl laboratories in the United States that perform diagnostic testing for health-
related reasons (i.e., with results returned to the patient or a health care practitioner) are regulated by CMS under the authority of CLIA. For CLIA to apply, testing must be carried out on a human specimen. CLIA certification is based on the level of complexity of testing that the laboratory performs, specificallyspecifical y (1) low (therefore, waived) complexity, (2) moderate complexity, and (3) high complexity. FDA is responsible for categorizing tests according to their level of complexity.22 CLIA requirements are used to evaluate a test'’s analytical validity, defined as the ability
ability of a test to detect or measure the analyte it is intended to detect or measure.23 Laboratories that perform moderate- and high-complexity testing must meet specific standards and requirements as a condition of certification, including proficiency testing (PT), patient test management, quality control, personnel qualifications, and quality assurance. All LDTs, including genetic tests offered as LDTs, are considered high-complexity tests under CLIA. All COVID-19 diagnostics would be considered to be high complexity tests under CLIA.
Al LDTs default to high-complexity under CLIA, and therefore may only be carried out by clinical laboratories
certified to do high-complexity testing.24 In addition, under the FDA’s COVID-19 diagnostics guidance, during the pendency of agency EUA review, al tests are to be performed only in high complexity clinical laboratories; upon EUA authorization, a test may be carried out in settings specified in the Letter of Authorization, including high or moderate complexity laboratories or
waived settings (for use at the point-of-care).25
How Are IVDs Regulated by the FDA During an Emergency Such as the COVID-19 Pandemic? as the Outbreak of COVID-19?
In certain public health or other emergency situations, the HHS Secretary may declare that existing circumstances justify the use of unapproved medical products for certain uses, or approved medical products for unapproved uses.626 This declaration facilitates access to not-yet-approved medical products in an expedited manner during certain emergency situations. In the
case of the COVID-19 disease, , then-HHS Secretary Azar determined that "there is a public health emergency and [declared]declared that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of the novel coronavirus."727 On the basis of this declaration, FDA issued an Emergency Use Authorization EUA authorizing the emergency use of the CDC-developed diagnostic test for COVID-19.8 The FDA also issued an EUA to the state of New York for an LDT developed by the state public health labs. To date, these are the only two EUAs for coronavirus diagnostics that the FDA has issued.9
How Does the Emergency Use Authority Apply to LDTs if They Are Generally Exempted28 The FDA issued the second diagnostics EUA under this authority, and the
first pursuant to its COVID-19 diagnostics guidance, to the New York State Department of Public
21 PHSA §353 [42 U.S.C. §263a]. 22 FDA, “CLIA Categorizations,” https://www.fda.gov/medical-devices/ivd-regulatory-assistance/clia-categorizations. 23 42 C.F.R. §493.1253(b)(2). 24 42 C.F.R. §493.17; and CDC, “T est Complexities,” https://www.cdc.gov/clia/test-complexities.html. 25 FDA, “Policy for Diagnostics T esting in Laboratories Certified to Perform High Complexity T esting under CLIA prior to Emergency Use Authorization for Coronavirus Disease-2019 during the Public Health Emergency,” May 11, 2020, p. 14, https://www.fda.gov/media/135659/download. 26 FFDCA §564 [21 U.S.C. §360bbb–3]. 27 FDA, “Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices,” https://www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices.
28 Letter of authorization from FDA to CDC, dated December 1, 2020, https://www.fda.gov/media/134919/download.
Congressional Research Service
5
Early Development and Regulation of Diagnostic Testing for COVID-19
Health for a real-time PCR test, and has since issued hundreds of EUAs for COVID-19
diagnostics, including for molecular, antigen and serology tests.29
How Does the Emergency Use Authority Apply to LDTs If They Are Generally Exempt from Premarket Requirements?
from Premarket Requirements?
During an emergency, all al laboratory-developed tests testing for the relevant pathogen (in this case, SARS-CoV-2) must eitherwere traditional y required to be approved, cleared, or authorized under an EUA to be legally carried out. As noted above, FDA generally EUA to be legal y marketed. Although FDA general y waives most regulatory requirements (e.g., premarket review) for LDTs in normal situations; nevertheless, LDTs may only be used , LDTs have nevertheless traditional y only been
able to be used clinical y with authorization (e.g., an EUA) during an emergency declaration pursuant to FFDCA Section 564.1030 That is, statutory requirements under FFDCA Section 564 applyhave applied to LDTs as they do to other medical products, and they applyhave applied to both commercial test kits—which are normallynormal y subject to FDA regulatory requirements—and to LDTs.31 However, notably, on August 19, 2020, HHS announced that, effective immediately, it
was rescinding al guidance, compliance manuals, website statements, or other informal issuances concerning FDA premarket review of LDTs. 32 The announcement applies to al LDTs—including COVID-19 LDTs—and states that FDA may not require premarket review for these tests absent a notice-and-comment rulemaking process. Per the announcement, premarket review includes PMA, premarket notification (510(k) notification), and EUA. HHS noted that laboratories may
voluntarily submit an EUA request, PMA, or 510(k) for LDTs.33
The EUA LDTs.
The EUA process is usuallyusual y used to expedite access to medical products that would otherwise need premarket approval or clearance in emergency situations. However, because premarket
approval requirements for LDTs are generallygeneral y waived through enforcement discretion by the agency, the EUA representsrepresented additional regulatory requirements for the use of an LDT in emergency situations. This is because, among other things, inIn the case of a communicable disease, the test result has implications beyond the individual being tested, and so a false negative result could have serious consequences for the community.11 Therefore, FDA stateshad stated that these tests need an EUA in an emergency prior to
clinical use as do other medical products. In contrast, for commercial test kits, the EUA represents an abbreviated mechanism that allowsal ows the unapproved product to be used without undergoing the FDA premarket review typically required (for a complex molecular test for a novel pathogen, that review would generally be a Premarket Approval, or PMA, for high-risk medical devices).
Despite a request from the Association of Public Health Laboratories (APHL) to FDA, the agency declined to exercise enforcement discretion with respect to LDTs that detect SARC-CoV-2 and diagnose COVID-19 and the requirement that they receive an EUA prior to use. APHL maintains that these labs
full premarket review typical y required.
Despite a request from the Association of Public Health Laboratories (APHL) to FDA in February 2020, the agency at that time declined to exercise enforcement discretion with respect to COVID-19 LDTs and the requirement that they receive EUA prior to clinical use. APHL maintained that clinical laboratories are regulated by CLIA, and that this regulatory oversight is sufficient.12 However, on February 29, 2020, FDA announced a new policy allowing certain laboratories that have developed and validated COVID-19 LDTs to begin to use the test clinically prior to it receiving an EUA 34
29 FDA Letter of Authorization to New York State Department of Public Health, initially dated February 29 2020, https://www.fda.gov/media/135661/download; and FDA, “ In Vitro Diagnostics EUAs,” https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas.
30 FFDCA §564 [21 U.S.C. §360bbb–3]. 31 FDA, “Emergency Use Authorization of Medical Products and Related Authorities,” p. 28, https://www.fda.gov/media/97321/download. 32 HHS, “Rescission of Guidances and Other Informal Issuances Concerning Premarket Review of Laboratory Developed T ests,” https://www.hhs.gov/coronavirus/testing/recission-guidances-informal-issuances-premarket -review-lab-tests/index.html.
33 For more information, see CRS Insight IN11548, HHS Announcement on FDA Premarket Review of Laboratory-Developed Tests (LDTs) . 34 360dx, “APHL Asks FDA to Make Own T ests as CDC Struggles to Provide SARS-CoV-2 T est Kits,” February 25, 2020, https://www.360dx.com/clinical-lab-management/aphl-asks-fda-make-own-tests-cdc-struggles-provide-sars-cov-
Congressional Research Service
6
link to page 12 link to page 12 link to page 11 link to page 11 Early Development and Regulation of Diagnostic Testing for COVID-19
However, partial y in response to these concerns, FDA issued its COVID-19 diagnostics guidance al owing certain clinical laboratories that have developed and validated COVID-19 tests, including LDTs, to begin to use the test clinical y prior to it receiving an EUA from the agency but after validation of the test and notification of the agency (see "“What Steps Did FDA Take to
Expand Testing Capacity in Response to the ProblemsIssues with CDC'’s Test?").13
”).35 The CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time Reverse Transcriptase (RT)-PCR Diagnostic Panel
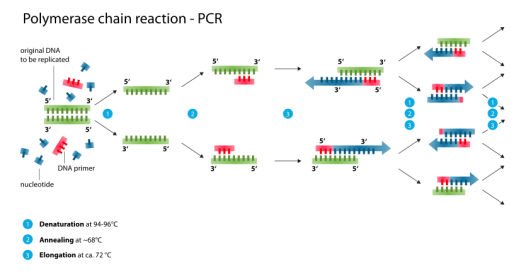
How Does the CDC'’s COVID-19 Diagnostic Test Work?
The diagnostic test developed by the CDC, calledcal ed the 2019-Novel Coronavirus (2019-nCoV) Real-Time Reverse Transcriptase (RT)-PCR Diagnostic Panel, is a complex molecular diagnostic
test that relies on generallygeneral y standard molecular biology laboratory techniques. SpecificallySpecifical y, the test uses a technique calledcal ed Polymerase Chain Reaction (PCR), a standard in vitro technique for amplification ofand identification of target DNA. Because the SARS-CoV-2 virus—the virus that causes COVID-19—is an RNA14 is an RNA36 virus, the RNA must be first reverse transcribed to generate copy DNA, or cDNA, which is then amplified (multiple copies are generated) using PCR. PCR relies on primers—very short single
stranded pieces of DNA that are complementary to and bind with specific regions of the viral genome and thus define the specific genomic region to be amplified. The test then relies on a probe, or a single-stranded piece of DNA that is chemicallychemical y or radioactively labelled, label ed, that can
bind to and thus detect the amplified target portion of the viral genetic material.
|
 |
|
CDC'
CDC’s original test used three sets of primers and probes: two to target specific regions of a designated gene within the SARS-CoV-2 viral genome, and a third that was specific to all al SARS-like coronaviruses (see " “What Quality Problems Did the CDC'’s Test Experience on Rollout to the State and Local Public Health Laboratories?”"). The test also includes a number of authorized
control samples, including a positive control for SARS-CoV-2 and a "“no template control"” to test for system contamination. Together, these controls help ensure that the test is functioning properly.
properly, and it was the performance of these controls that provided the first indication that the
CDC test was contaminated.37
What Type of IVD Is the CDC'’s Test and Who May Carry It Out?
The CDC's test is being developed by the agency as a test kit and is generally ’s test is a kit and was initial y authorized to be distributed to state and local public health laboratories to augment public health testing capacity. The test received an EUA from the 2-test-kits?trendmd-shared=0&utm_medium=T rendMD&utm_campaign=0&utm_source=T rendMD#.XmKIwqhKiUm.
35 See generally FDA, “Policy for Coronavirus Disease-2019 T ests During the Public Health Emergency (Revised),” originally dated February 29, 2020, https://www.fda.gov/media/135659/download. 36 RNA stands for ribonucleic acid. RNA is genetic material with a slightly different chemical composition from DNA, or deoxyribonucleic acid.
37 Washington Post, “Summary of the Findings of the Immediate Office of the General Counsel’s Investigation Regarding CDC’s Production of COVID-19 T est Kits,” https://www.washingtonpost.com/context/summary-of-the-findings-of-the-immediate-office-of-the-general-counsel-s-investigation-regarding-cdc-s-production-of-covid-19-test-kits/a750fbf7-9a4f-4062-8fcc-c6cf42600578/.
Congressional Research Service
7
Early Development and Regulation of Diagnostic Testing for COVID-19
health laboratories to augment public health testing capacity. The test received an EUA from the FDA on February 4, 2020, under which "“authorized laboratories" may receive and” could carry out the test despite the fact that it is not FDA-approved or FDA-cleared, and that it does not meet all al related regulatory requirements for marketing.1538 The EUA notesnoted that "“[t]esting is limited to qualified laboratories designated by CDC and, in the United States, certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), (; 42 U.S.C. §263a), to perform high complexity tests."16”39 CDC-qualified laboratories with a CLIA certification for high-complexity testing able to
receive the test kits includeincluded U.S. state and local public health laboratories, Department of Defense (DOD) laboratories, and select international laboratories. The public40 Public health laboratories must verify the test themselves prior to using it, and are currently required use, and were required initial y to send presumptive positive cases back to the CDC in Atlanta for confirmatory testing by the agency. Subsequent amendments to the EUA for the CDC test expanded authorized laboratories to include al high-complexity
clinical laboratories.41
What Quality Problems Did the CDC’cases back to the CDC in Atlanta for confirmatory testing by the agency.
What Is the Role of the Commercial Manufacturer IDT in the Development and Distribution of the CDC's Test Kit?
The Trump Administration had estimated that approximately 1 million tests would be broadly available imminently, facilitated by leveraging manufacturing of the CDC test kit by a private company, Integrated DNA Technologies (IDT), a manufacturer that has been working with CDC.17 According to HHS Secretary Azar on March 5, 2020, IDT will send the CDC test kit to "hospitals, other labs around the country, commercial, public health … labs" by the end of the week of March 2.18 The test kit being manufactured by IDT, identical to the CDC test kit and referred to as "IDT 2019-novel coronavirus kit," is being qualified by CDC in lots, and FDA reports that these CDC-qualified, IDT-manufactured test kits are covered by the CDC's EUA authorization of February 4, 2020.19
FDA notes that if a lab purchases a test kit from IDT, the laboratory does not need its own EUA to carry it out, but that "[t]esting using CDC's EUA-authorized protocol and CDC qualified lots of reagents is considered to be testing done under the CDC's EUA. Labs performing such testing should follow any applicable conditions set forth in the EUA."20 LabCorp, a large commercial laboratory, is already reporting that it can perform the CDC test "if needed to meet testing demand" and that the test is only for use with "authorized specimens collected from individuals who meet CDC criteria for COVID-19 testing."21 IDT is manufacturing test kits in two sizes, with the largest having a capacity of 500 reactions (approximately 250 individual patients).22
What Quality Problems Did the CDC's Test Experience on Rollout to the State and Local Public Health Laboratories?
As noted above, the CDC'’s test kit used three sets of probes and primers—or reagents—to detect and identify viral DNA beyond that specific to COVID-19. One of these reagents, the one meant to detect any SARS-like coronavirus including SARS-CoV-2, was returning inconclusive results. In response
To address this, the CDC validated a new protocol for theirits test that allowsal owed it to be run excluding the faulty reagent, running the test with only the other two diagnostic components. CDC hashad the authority to modify the test through enforcement discretion granted by FDA.2342 The agency determined that the exclusion of this reagent does not affect the accuracy or the sensitivity and specificity of the test.
Certain laboratories have continued to experience problems running the test, even when using the modified
protocol, with at least one laboratory reporting that the first reagent was also returning inconclusive results.43 This problem severely limited the state and local public health laboratories'’ ability
to carry out the CDC'’s test.
In response to these issues, the New York State Department of Public Health requested and was granted the FDA'’s second EUA for its own laboratory-developed test, the New York SARS-CoV-2 Real-time RT-PCR Diagnostic Panel.24 Testing is 44 Testing was initial y limited under the EUA to two laboratories in New York—the Wadsworth Center, New York State Department of Public Health,
and the New York City Department of Health and Mental Hygiene, Public Health Laboratories. New York was one of the states that had continued difficulty implementing CDC'’s original test
kit, even with the modified protocol.
In response, CDC is also manufacturing
38 EUA Letter from FDA to CDC, originally dated February 4, 2020, https://www.fda.gov/media/134919/download. 39 Ibid. 40 CDC, “Shipping of CDC 2019 Novel Coronavirus Diagnostic T est Kits Begins,” https://www.cdc.gov/media/releases/2020/p0206-coronavirus-diagnostic-test-kits.html.
41 Ibid. 42 CDC, “Revision to T est Instructions: CDC 2019 Novel Coronavirus (nCoV) Real-T ime RT -PCR Diagnostic Panel (EUA200001),” February 26, 2020, https://www.aphl.org/Materials/Signed_CDC_Letter_to_PHLs-N3_Removal_Instructions_26Feb2020.pdf.
43 NPR, “CDC Report: Officials Knew Coronavirus T est Was Flawed But Released It Anyway,” November 6, 2020, https://www.npr.org/2020/11/06/929078678/cdc-report-officials-knew-coronavirus-test-was-flawed-but-released-it-anyway.
44 FDA Letter to NYSDOH, originally dated February 29, 2020, https://www.fda.gov/media/135661/download.
Congressional Research Service
8
Early Development and Regulation of Diagnostic Testing for COVID-19
CDC manufactured new test kits after reportedly resolving the manufacturing issue that affected the original test kit. This time, however, CDC is manufacturingmanufactured test kits with only the two reagents that were unaffected by the quality issue—that are specific to SARS-CoV-2— and those kits are beingwere made available to qualified CDC
labs through the International Reagent Resource.25,26 These test kits are expected to result in the public health laboratories having capacity to test up to 75,000 patients.27
(IRR).45,46
Some believe that the CDC'’s choice to develop and mitigate quality problems with its own COVID-19 diagnostic when an accepted diagnostic was available through the World Health Organization (WHO), which was efficiently distributing a German-developed test globally global y early in the outbreak, was a decision that cost the United States time in its response to the virus's ’s
introduction and spread in the country.2847 Some speculated about the use of the third reagent—that was to detect SARS-like coronaviruses— and whether it had been strictly necessary if the test was still stil accurate at diagnosing COVID-19 without that reagent included in the test at all, or, and if it had instead overcomplicated the test.2948 In general, there have been questions raised about the CDC'’s handling of the development and distribution of its test, and its response to the quality problems that occurred, and the impact this may have had on the country's ability ’s ability to detect community spread of the disease before it occurred more widely.30
49 As previously
noted, HHS conducted its own internal review of the CDC’s manufacture of its test, and the HHS
OIG is currently investigating this issue, as wel .
What Steps Did FDA Take to Expand Testing Capacity in Response to the ProblemsIssues with CDC'’s Test? FDA’s COVID-19 diagnostic guidance was published at least partial y in response tos Test?
On February 29, 2020, as problems with the rollout of the CDC-developed diagnostic test continued, FDA announced a new policy to . As noted, the policy was meant to
immediately leverage LDTsclinical laboratory tests, including LDTs, developed in high-complexity commercial, reference, and clinical laboratories nationwide to expand testing capacity. SpecificallySpecifical y, the new agency guidance allowsal owed CLIA-certified high-complexity laboratories that havehad developed and validated their own COVID-19 diagnostics to use the tests while the
laboratory is preparing, and FDA is reviewing, their EUA submission.31,32
The 50,51
The initial FDA guidance statesstated that laboratories havehad 15 days after validating their test and notifying the agency to submit an EUA application to FDA, and the guidance recommends recommended
confirming the test'’s first five negative and positive results against an EUA-authorized
45 GenomeWeb, Johnson, Madeleine, “CDC Revises SARS-CoV-2 Assay Protocol; Surveillance T esting on T rack to Start Next Week,” February 28, 2020, https://www.genomeweb.com/pcr/cdc-revises-sars-cov-2-assay-protocol-surveillance-testing-track-start-next-week#.Xl63kqhKiUk.
46 “T he International Reagent Resource (IRR) was established by the Centers for Disease Control and Prevention (CDC) to provide registered users with reagents, tools and information for studying and detection of Influenza Virus,” see https://www.internationalreagentresource.org/About/IRR.aspx. 47 Sheridan, Cormac, “Coronavirus and the Race to Distribute Reliable Diagnostics,” Nature, February 19, 2020, https://www.nature.com/articles/d41587-020-00002-2.
48 ProPublica, “Key Missteps at the CDC Have Set Back its Ability to Detect the Potential Spread of Coronavirus,” February 28, 2020, https://www.propublica.org/article/cdc-coronavirus-covid-19-test.
49 Politico, “How testing failures allowed the coronavirus to sweep the United States,” March 6, 2020, https://www.politico.com/news/2020/03/06/coronavirus-testing-failure-123166. 50 GenomeWeb, “FDA Provides SARS-CoV-2 T est Validation Guidance for High-Complexity Labs Seeking EUA,” March 3, 2020, https://www.genomeweb.com/pcr/fda-provides-sars-cov-2-test-validation-guidance-high-complexity-labs-seeking-eua#.Xl7JFqhKiUk.
51 FDA, originally titled “Policy for Diagnostics T esting in Laboratories Certified to Perform High Complexity T esting s first five negative and positive results against an EUA-authorized diagnostic. According to FDA, it "does not intend to object to the use of these tests for clinical testing while the laboratories are pursuing an EUA with the FDA. Importantly, this policy only applies to laboratories that are certified to perform high-complexity testing consistent with requirements under Clinical Laboratory Improvement Amendments."33 The guidance includes detailed information about FDA's expected methods for test validation.
Pursuant to this FDA guidance, on March 5, 2020, LabCorp announced that it had begun offering its LDT, the LabCorp 2019 Novel Coronavirus (COVID-19), NAA test "for ordering by physicians or other authorized healthcare providers anywhere in the U.S.," and that it is currently pursuing an EUA with the agency for the test.34 This test is to take between three to four days to return results, and a health care provider must order and authorize it, and obtain the required specimen from the patient. Under the FDA's new guidance, Quest has also announced that it will begin testing for coronavirus with its own LDT, beginning March 9, 2020, and that it plans to pursue an EUA with the agency within 15 days, as required by the guidance.35
Author Contact Information
Footnotes
| 1. |
For more information about the domestic response to the novel coronavirus, see CRS Report R46219, Overview of U.S. Domestic Response to Coronavirus Disease 2019 (COVID-19). |
| 2. |
"U.S. agency investigating production of faulty coronavirus test kits," March 1, 2020, https://www.reuters.com/article/us-china-health-test-kits/u-s-agency-investigating-production-of-faulty-coronavirus-test-kits-idUSKBN20P09Y. |
| 3. |
See generally FDA, "Policy for Diagnostics Testing in Laboratories Certified to Perform High Complexity Testing under CLIA prior to Emergency Use Authorization for Coronavirus Disease-2019 during the Public Health Emergency," February 29, 2020, https://www.fda.gov/media/135659/download. |
| 4. |
The term medical device is defined in FFDCA §201(h) [21 U.S.C. §321(h)]. |
| 5. |
PHSA §353; 42 U.S.C. §263a. |
| 6. |
FFDCA §564 [21 U.S.C. §360bbb–3]. |
| 7. |
FDA, "Policy for Diagnostics Testing in Laboratories Certified to Perform High Complexity Testing under CLIA prior to Emergency Use Authorization for Coronavirus Disease-2019 during the Public Health Emergency," February 29, 2020, https://www.fda.gov/media/135659/download, page 1. |
| 8. |
Letter of authorization from FDA to CDC, advising of the authorization for emergency use of the CDC-developed diagnostic test, available at "Novel coronavirus (COVID-2019) EUA Information" on the FDA web page "Emergency Use Authorization," https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization. |
| 9. |
FDA, Emergency Use Authorization, https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization. |
| 10. |
21 U.S.C. §360bbb–3. |
| 11. |
FDA, "Information for Laboratories Implementing IVD Tests Under an EUA," https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/information-laboratories-implementing-ivd-tests-under-eua. |
| 12. |
360dx, "APHL Asks FDA to Make Own Tests as CDC Struggles to Provide SARS-CoV-2 Test Kits," February 25, 2020, https://www.360dx.com/clinical-lab-management/aphl-asks-fda-make-own-tests-cdc-struggles-provide-sars-cov-2-test-kits?trendmd-shared=0&utm_medium=TrendMD&utm_campaign=0&utm_source=TrendMD#.XmKIwqhKiUm. |
| 13. |
|
| 14. |
RNA stands for ribonucleic acid. RNA is genetic material with a slightly different chemical composition from DNA, or deoxyribonucleic acid. |
| 15. |
EUA Letter from FDA to CDC, dated February 4, 2020, https://www.fda.gov/media/134919/download. |
| 16. |
Ibid. |
| 17. |
RAPS, "FDA's Hahn Addresses Coronavirus Test Capacity, Drug Shortages at Senate Hearing," March 3, 2020, https://www.raps.org/news-and-articles/news-articles/2020/3/fdas-hahn-addresses-coronavirus-test-capacity-dr. |
| 18. |
Secretary of HHS Alex Azar, Coronavirus Press Briefing, March 5, 2020, https://www.c-span.org/video/?470068-2/secretary-azar-federal-health-officials-coronavirus-briefing. |
| 19. |
Stenzel, Timothy, FDA Office of In Vitro Diagnostics Webinar, "Policy for Diagnostics Testing in Laboratories Certified to Perform High Complexity Testing under CLIA prior to Emergency Use Authorization for Coronavirus Disease-2019 during the Public Health Emergency" Immediately in Effect Guidance," March 2, 2020; see slide 13, https://www.fda.gov/media/135707/download. |
| 20. |
FDA, "FAQs on Diagnostic Testing for SARS-CoV-2," https://www.fda.gov/medical-devices/emergency-situations-medical-devices/faqs-diagnostic-testing-sars-cov-2. |
| 21. |
LabCorp, "Information from LabCorp about Coronavirus Disease 2019 (COVID-19)," March 5, 2020, https://www.labcorp.com/information-labcorp-about-coronavirus-disease-2019-covid-19. |
| 22. |
IDT, https://www.idtdna.com/pages/landing/coronavirus-research-reagents. |
| 23. |
CDC, "Revision to Test Instructions: CDC 2019 Novel Coronavirus (nCoV) Real-Time RT-PCR Diagnostic Panel (EUA200001)," February 26, 2020, https://www.aphl.org/Materials/Signed_CDC_Letter_to_PHLs-N3_Removal_Instructions_26Feb2020.pdf. |
| 24. |
FDA Letter to NYSDOH, February 29, 2020, https://www.fda.gov/media/135661/download. |
| 25. |
GenomeWeb, Johnson, Madeline, "CDC Revises SARS-CoV-2 Assay Protocol; Surveillance Testing on Track to Start Next Week," February 28, 2020, https://www.genomeweb.com/pcr/cdc-revises-sars-cov-2-assay-protocol-surveillance-testing-track-start-next-week#.Xl63kqhKiUk. |
| 26. |
"The International Reagent Resource (IRR) was established by the Centers for Disease Control and Prevention (CDC) to provide registered users with reagents, tools and information for studying and detection of Influenza Virus," see https://www.internationalreagentresource.org/About/IRR.aspx. |
| 27. |
CDC, "Coronavirus Disease 2019 (COVID-19) Situation Summary," Updated March 3, 2020, https://www.cdc.gov/coronavirus/2019-ncov/summary.html. |
| 28. |
Sheridan, Cormac, "Coronavirus and the Race to Distribute Reliable Diagnostics," Nature, February 19, 2020, https://www.nature.com/articles/d41587-020-00002-2. |
| 29. |
ProPublica, "Key Missteps at the CDC Have Set Back its Ability to Detect the Potential Spread of Coronavirus," February 28, 2020, https://www.propublica.org/article/cdc-coronavirus-covid-19-test. |
| 30. |
Politico, "How testing failures allowed the coronavirus to sweep the United States," March 6, 2020, https://www.politico.com/news/2020/03/06/coronavirus-testing-failure-123166. |
| 31. |
GenomeWeb, "FDA Provides SARS-CoV-2 Test Validation Guidance for High-Complexity Labs Seeking EUA," March 3, 2020, https://www.genomeweb.com/pcr/fda-provides-sars-cov-2-test-validation-guidance-high-complexity-labs-seeking-eua#.Xl7JFqhKiUk. |
| 32. |
FDA, "Policy for Diagnostics Testing in Laboratories Certified to Perform High Complexity Testing under CLIA prior to Emergency Use Authorization for Coronavirus Disease-2019 during the Public Health Emergency," February 29, 2020, https://www.fda.gov/media/135659/download. |
| 33. |
FDA, "Coronavirus (COVID-19) Update: FDA Issues New Policy to Help Expedite Availability of Diagnostics," press release with link to guidance, February 29, 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-new-policy-help-expedite-availability-diagnostics. See also CRS In Focus IF11389, FDA Regulation of Laboratory-Developed Tests (LDTs). |
| 34. |
LabCorp, "Information from LabCorp about Coronavirus Disease 2019 (COVID-19)," March 5, 2020, https://www.labcorp.com/information-labcorp-about-coronavirus-disease-2019-covid-19. |
| 35. |
QuestDiagnostics, "Quest Diagnostics to Launch Coronavirus Disease 2019 (COVID019) Test," March 5, 2020, https://newsroom.questdiagnostics.com/2020-03-05-Quest-Diagnostics-to-Launch-Coronavirus-Disease-2019-COVID-19-Test. |