The Agriculture appropriations bill—formally known as the Agriculture, Rural Development, Food and Drug Administration, and Related Agencies Appropriations Act—funds the Food and Drug Administration (FDA) and the U.S. Department of Agriculture (USDA), excluding the U.S. Forest Service. This includes funding for FDA and USDA's Food Safety and Inspection Service (FSIS), the two primary federal agencies responsible for overseeing the safety of the nation's food supply.
In March 2018, Congress enacted the FY2018 agriculture appropriation as part of the Consolidated Appropriations Act, 2018 (P.L. 115-141, Division A). Both the House and the Senate Appropriations Committees have reported Agriculture appropriations bills for FY2019 (H.R. 5961, S. 2976). The Senate amended and passed its version as Division C of a four-bill minibus (H.R. 6147). The final appropriation for FY2019 is pending, and current funding is authorized under a continuing resolution (P.L. 115-245) through December 7, 2018. The enacted FY2018 appropriation and both the FY2019 House-reported bill and the Senate-passed bill include funding for food safety programs and related activities at FDA and USDA.
This report provides a brief overview of the FY2018 and FY2019 appropriations that address food safety activities at FDA and FSIS. It does not specifically address funding levels for other federal agencies or other USDA agencies that may play a role in ensuring the safety of the nation's food supply.1 For a more general analysis of the FY2018 appropriations for agriculture, see CRS Report R45128, Agriculture and Related Agencies: FY2018 Appropriations; and for FY2019, see CRS Report R45230, Agriculture and Related Agencies: FY2019 Appropriations.
Introduction
Numerous federal, state, and local agencies share responsibilities for regulating the safety of the U.S. food supply.2 Federal responsibility for food safety rests primarily with FDA and FSIS. FDA, an agency of the Department of Health and Human Services (HHS), is responsible for ensuring the safety of the majority of all domestic and imported food products (except for meat and poultry products).3 FSIS, an agency at USDA, regulates most meat, poultry, and processed egg products.4 Roughly speaking, FSIS regulates about 10%-20% of the U.S. food supply, while FDA is responsible for the remaining 80%-90%.5
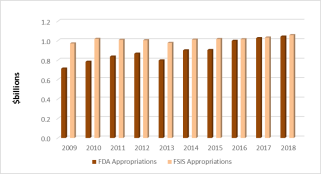
Combined appropriations covering food safety activities at both FDA and USDA totaled nearly $2.1 billion in FY2018, which is roughly split between the two agencies (Figure 1, Table 1). This funding distribution reflects greater increases in congressional appropriations for FDA compared with FSIS since FY2011. The enacted FY2018 appropriation for FDA's food safety activities provided $1,041.6 million. For FSIS, the FY2018 appropriation provided $1,056.8 million.
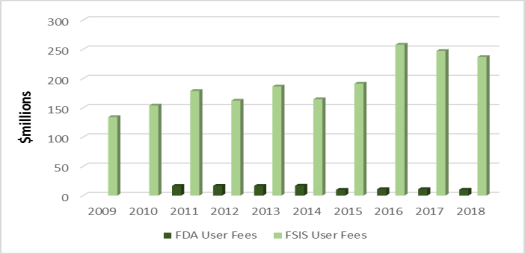
Annual appropriations for FDA and USDA are augmented by existing (currently authorized) user fees. Collected user fees differ between the two agencies: At FDA, user fees authorized under the FDA Food Safety Modernization Act (FSMA, P.L. 111-353) have generated between $10 million and $20 million annually in recent years; at FSIS, authorized user fees have generated between $180 million and $250 million annually (Figure 2). Staffing levels also differ between the two agencies: FSIS staff number around 9,200 full-time equivalents (FTEs), while FDA's food-related staff (whose activities extend beyond food safety) number about 3,900 FTEs (Table 1).
|
Figure 1. Appropriations for Federal Food Safety Activities, FY2009-FY2018
|
![]() |
|
Source: CRS, using data from FDA and FSIS (see Table 1). Data are not adjusted for inflation.
|
|
Figure 2. User Fees for Federal Food Safety Activities, FY2009-FY2018
|
 |
|
Source: CRS, using data from FDA and FSIS (see Table 1). Data are not adjusted for inflation.
Notes: FDA user fees include food and feed recall fees, food reinspection fees, fees collected under the voluntary qualified importer program, and export certification fees. FSIS user fees include fees for meat, poultry, and egg products inspection; fees for the cost of national laboratory accreditation programs; and trust funds.
|
Table 1. Food Safety Enacted Appropriations and Agency-Reported Funding Levels
|
Agency/Year
|
Federal Full-Time Equivalents
|
Enacted Appropriation ($ millions)
|
Total Agency-Reported Program Level (incl. fees and other funding)b ($ millions)
|
|
HHS Food and Drug Administration (FDA) "Foods Program"a
|
|
FY2009 (P.L. 111-8)
|
2,995
|
648.7
|
NA
|
|
FY2010 (P.L. 111-80)
|
3,387
|
782.9
|
NA
|
|
FY2011 (P.L. 112-10)
|
3,605
|
837.4
|
NA
|
|
FY2012 (P.L. 112-55)
|
3,611
|
978.7
|
NA
|
|
FY2013 (P.L. 113-6)c
|
3,642
|
796.6
|
NA
|
|
FY2014 (P.L. 113-76)
|
3,650
|
900.3
|
NA
|
|
FY2015 (P.L. 113-235)
|
3,667
|
903.4
|
913.8
|
|
FY2016 (P.L. 114-131)
|
3,841
|
987.3
|
1,040.8
|
|
FY2017 (P.L. 115-31)
|
3,905
|
1,025.5
|
1,025.5
|
|
FY2018 (P.L. 115-141)
|
3,939
|
1,041.6
|
1,033.1
|
|
FY2019 (President's Budget)
|
3,939
|
1,029.9
|
1,040.7
|
|
FY2019 (H.R. 5961)
|
NA
|
1,039.7
|
NA
|
|
FY2019 (S. 2976/S.Amdt. to H.R. 6147)
|
NA
|
1,052.3
|
NA
|
|
USDA Food Safety and Inspection Service (FSIS)
|
|
FY2009 (P.L. 111-8)
|
9,343
|
971.6
|
1,110.1
|
|
FY2010 (P.L. 111-80)
|
9,401
|
1,018.5
|
1,152.6
|
|
FY2011 (P.L. 112-10)
|
9,465
|
1,008.5
|
1,168.4
|
|
FY2012 (P.L. 112-55)
|
9,351
|
1,004.4
|
1,170.8
|
|
FY2013 (P.L. 113-6)c
|
9,158
|
977.3
|
1,162.0
|
|
FY2014 (P.L. 113-76)
|
8,933
|
1,010.7
|
1,170.0
|
|
FY2015 (P.L. 113-235)
|
8,938
|
1,016.5
|
1,206.7
|
|
FY2016 (P.L. 114-131)
|
9,160
|
1,014.9
|
1,284.1
|
|
FY2017 (P.L. 115-31)
|
9,243
|
1,032.1
|
1,288.0
|
|
FY2018 (P.L. 115-141)
|
9,054
|
1,056.8
|
1,266.2
|
|
FY2019 (President's Budget)
|
9,224
|
1,032.3
|
1,274.1
|
|
FY2019 (H.R. 5961)
|
NA
|
1,049.3
|
NA
|
|
FY2019 (S. 2976/S.Amdt. to H.R. 6147)
|
NA
|
1,049.3
|
NA
|
Sources: CRS, from enacted appropriations and from annual agency budget justifications for FDA (http://www.fda.gov/AboutFDA/ReportsManualsForms/Reports/BudgetReports/default.htm) and FSIS (http://www.obpa.usda.gov/explan_notes.html). Italicized data and text are not enacted.
Notes: NA = Not available. Data for FDA before FY2015 are not available to make comparisons.
a. Reflects appropriations for Center for Food Safety and Applied Nutrition and related field activities in the Office of Regulatory Affairs. FDA's "Foods Program" includes all food activities, not only food safety.
b. For FDA, reflects available funding for total food safety activities across all FDA programs as well as user fees. For FSIS, includes other federal and non-federal (fees and trust funds) funding.
c. FY2013 totals represent each agency's FY2013 sequestration operating plans.
In addition to setting budgetary amounts, Agriculture appropriations bills may also include policy-related provisions that direct how the executive branch should carry out a specific appropriation. These provisions may have the force of law if they are included in the text of the appropriation, but their effect is generally limited to the fiscal year indicated.
The explanatory statement that accompanies the final appropriation, and the House and the Senate report language that accompanies the committee-reported bills, may also provide policy instructions. These documents do not have the force of law but often explain congressional intent, which the agencies are expected to follow. The committee reports and explanatory statement may need to be read together to capture all of the congressional intent for a given fiscal year. According to the FY2018 Explanatory Statement, "The explanatory statement is silent on provisions that were in both the House Report (H.Rept. 115-232) and Senate Report (S.Rept. 115-131) that remain unchanged by this agreement, except as noted in this explanatory statement.... The House and Senate report language that is not changed by the explanatory statement is approved and indicates congressional intentions. The explanatory statement, while repeating some report language for emphasis, does not intend to negate the language referred to above unless expressly provided herein."6
FDA Food Safety Activities
FDA's Foods Program covers the agency's food safety activities, as well as certain other food-related programs. The program plays a major food safety role, ensuring that the nation's food supply, quality of foods, food ingredients, and dietary supplements (and also cosmetic products) are safe, sanitary, nutritious, wholesome, and properly labeled. FDA's Foods Program budget accounts for roughly one-third of FDA's total appropriation. FDA's total budget for food safety programs and activities extends beyond the agency's Foods Program to encompass other food and veterinary medicine programs at FDA while also including aspects of other FDA program areas covering food additives, antimicrobial resistance, and nutrition labeling.7
Recent Enacted Appropriations
In recent years, congressional appropriators have increased funding for FDA's Foods Program, raising funding from $837.4 million in FY2011 to $1,041.6 million in FY2018. Increased funding over this period followed the enactment of comprehensive food safety legislation in the 111th Congress as part of FSMA. FSMA was the largest expansion of FDA's food safety authorities since the 1930s, and FDA is still actively engaged in implementing the law and its regulations.8 Over the past few decades, FDA has also had to adapt to the increasing variety and complexity of the U.S. food supply, including rising demand for products produced outside the United States, as well as other developments, including emerging microbial pathogens, natural toxins, and technological innovations in production and processing.
Although Congress authorized funds to be appropriated in FSMA, it did not provide the full funding needed for FDA to perform these expanded activities under the law, according to FDA.9 Prior to enactment, the Congressional Budget Office (CBO) estimated that fully implementing FSMA could increase net federal spending subject to appropriation by about $1.4 billion over a five-year period (FY2011-FY2015).10 This cost estimate covered activities at FDA and other federal agencies and did not include offsetting revenue from the collection of new user fees authorized under FSMA.
Following FSMA's enactment, FDA officials continued to emphasize the need for additional FDA funding.11 Food industry groups also called for increased budget authority for FDA to fully implement FSMA, at levels requested by the Administration, in order to maintain consumer confidence.12 State agriculture officials and representatives of the National Association of State Departments of Agriculture (NASDA) further pushed for full FSMA funding to support implementation by front-line state officials.13 Public health and consumer safety groups, as well as victims of food-borne illnesses, have also continued to call for additional food safety funding.14
Since FSMA became law in 2011, congressional appropriators have increased funding for the FDA Foods Program by $204.3 million—an increase of about 24% between FY2011 and FY2018—largely in an effort to support FDA's implementation of FSMA. Currently, FDA's annual appropriation to conduct its food safety oversight activities is roughly similar to that at FSIS (Figure 1, Table 1). Cumulative increases to the agency's budget authority to address food safety activities and FSMA implementation since FY2011 have totaled more than $300 million, according to FDA (Table 2).
FSMA also authorized an increase in FDA staff, which was expected to reach 5,000 by FY2014.15 With 3,900 food safety FTEs in FY2018, FDA has fallen short of the goals set forth in FSMA.
Of the total amount appropriated to FDA, the enacted FY2018 act specifically provides that $1.5 million be used to conduct oversight of FDA's programs and operations by HHS's Office of Inspector General and that $1.5 million be used for FDA and USDA to coordinate public education activities regarding crop biotechnology and food and animal feed ingredients derived from biotechnology. The latter allocation originated in the House committee bill.
Table 2. FDA Food Safety Program Funding and Changes to FSMA, FY2011-FY2018
|
|
| Total FDA Food Safety Changes |
|
Net FSMA Changes
|
Explanation
|
|
FY2011
|
|
|
Increases per conference documents provided to FDA
|
|
FY2012
|
|
|
FSMA increases per conference agreement (p. 185)
|
|
FY2013a
|
|
|
FSMA increases per S.Rept. 112-163 (p. 77), less permanent base reduction due to sequestration
|
|
FY2014
|
|
|
FSMA increases per S.Rept. 113-46 (p. 79)
|
|
FY2015
|
|
|
Food safety increases per Congressional Record, December 11, 2014 (p. H9314)
|
|
FY2016
|
|
|
Explanatory text, Division A, Agriculture, Rural Development, Food and Drug Administration, and Related Agencies Appropriations Act, 2016 (p. 29)
|
|
FY2017
|
|
|
Provided an increase of $38.7 million for food safety activities, of which $35.7 million was to support FSMA implementation (Congressional Record, May 3, 2017, Book II, H3334).
|
|
FY2018
|
|
|
According to FDA, increases for various food safety activities specified in report language (e.g., covering FDA oversight activities, lab testing, educational programs, and other activities).
|
|
Subtotal
|
|
|
|
Congressional appropriations are augmented by existing industry-paid user fees authorized by FSMA. FDA user fees include food and feed recall fees, food reinspection fees, voluntary qualified importer program fees, and fees for certain periodic activities involving reinspection, recall, and export certification.16 Since FSMA was enacted, these fees have generated between $10 million and $20 million annually (Figure 2). The Obama Administration requested authorization of new user fees totaling nearly $170 million annually, but congressional appropriators did not accept its proposals.17 User fees are generally established in law by the authorizing committees and not by appropriators.
Selected Provisions in the FY2018 Appropriations
Language in the enacted FDA appropriation for FY2018, along with other statements in the House and the Senate committee reports, continues to address certain FSMA implementation issues. The enacted FY2018 appropriation act states that "none of the funds made available by this or any other Act" may be used to implement FSMA requirements regarding the regulation of the production, distribution, sale, or receipt of dried spent grain byproducts of the alcoholic beverage production process.18 Such byproducts are often used as animal feed. As part of FSMA's produce regulations, both the House and the Senate committee reports direct FDA to distinguish between grape varieties intended for wine production and grapes consumed raw. The Senate committee also expressed concerns about FSMA regulations on animal feed derived from cotton ginning and cottonseed.19 It also directs FDA to provide further clarification to small farms on the requirements for FSMA compliance and to offer technical assistance to facilitate compliance among small farms. The House committee report further states that "it is the intent of Congress for FDA to ensure an even playing field in the application of FSMA regulations as it relates to both domestic and imported producers, processors, and manufacturers of food and animal feed."20 It also requests that FDA provide the committee with a timeline of all activities associated with the investigation into the illnesses associated with imported pet food.
In addition to FSMA implementation, the enacted FY2018 appropriation addresses other food safety issues under FDA's jurisdiction, including issues regarding fish and seafood safety, the use of biotechnology, standards of identity, and food product labeling. Table 3 and Table 4 provide a summary of selected provisions.
On issues regarding fish and seafood safety, for example, both the House and the Senate committees direct FDA to review its final seafood advisory for pregnant and nursing women. The House committee further provides $2.8 million to support the development of appropriate lab methods to detect evidence of seafood decomposition. The Senate committee encourages FDA to increase funding for research into Vibrio illnesses associated with the consumption of raw molluscan shellfish. The enacted FY2018 act prohibits FDA from allowing the "introduction into interstate commerce of any food that contains genetically engineered salmon until the FDA publishes final labeling guidelines."
Both committees direct FDA to continue to monitor fraud in imported olive oil. The Senate committee encourages FDA to respond to a 2012 citizen petition requesting a standard of identity for olive oil and olive pomace oil, while the House committee directs FDA to update its 2014 survey of olive oil products. The House committee report also includes language directing FDA to develop a dairy-specific standard of identity and guidance in accordance with the Dairy Pride Act (H.R. 778/S. 130). It also directs FDA to define natural so that there is a uniform national standard for the labeling claims. The Senate committee directs FDA to address concerns about misleading maple syrup marketing.21
The enacted appropriation and committee bills also contain other policy riders for FDA's Foods Program that are not necessarily related to the agency's food safety activities. For example, the enacted FY2018 appropriation restricts FDA's ability to deem a food containing partially hydrogenated oils to be unsafe or adulterated and prohibits the agency from using appropriated funds to "develop, issue, promote or advance" regulations or "final guidance applicable to food manufacturers for long-term population-wide sodium reduction."22 Provisions in the House committee report also address menu labeling requirements and the nutrition facts label, noting that FDA had not issued final guidance regarding the definition of dietary fiber and labeling of added sugars.23
Selected Provisions in the FY2019 Appropriations
The House-reported bill would appropriate $1,039.7 million for FY2019, which is less than the Senate-passed bill's appropriation of $1,052.3 million (Table 1). By comparison, the Administration's FY2019 budget request recommends $1,029.9 million for FDA's Foods Program.24 Congressional appropriations would be augmented by FSMA-authorized user fees. The Administration is not proposing any new user fees for FDA's Foods Program.
Of the total amount appropriated to FDA, both the House and the Senate bills would direct agency spending on specific activities. The Senate-passed bill would require that no less than $15 million be used for inspections of foreign seafood manufacturers and field examinations of imported seafood. It would also require that $1.5 million be used to conduct oversight of FDA's programs and operations by HHS's Office of Inspector General. The House-reported bill would also direct $1.5 million for HHS oversight activities and that $3 million be used by FDA and USDA to coordinate public education activities on the safety and benefits of crop biotechnology.
For FDA, the House and Senate FY2019 appropriation bill language, along with other statements in the House and the Senate committee reports, address other food safety issues under FDA jurisdiction (Table 3). Similar to those enacted in the FY2018 appropriations act, these include issues addressing FSMA implementation, fish and seafood safety and fraud, the use of biotechnology, standards of identity, and food product labeling. The House and the Senate appropriation and committee bills also contain a number of policy riders for FDA's Foods Program that are not necessarily related to the agency's food safety activities but address certain agency nutritional guidance and regulations.
Table 3 and Table 4 provide a summary of selected provisions in the proposed FY2019 appropriation bills and committee reports with comparisons with those enacted for FY2018.
FSIS Food Safety Activities
USDA's FSIS is responsible for inspecting U.S. supplies of meat, poultry, and processed egg products to ensure that they are safe, wholesome, and properly labeled and packaged. The agency's Meat and Poultry Inspection Program conducts continual inspections at federal meat and poultry plants. FSIS also ensures that meat and poultry products imported to the United States are produced under standards equivalent to U.S. inspection standards and facilitates the certification of regulated products.25
Selected Provisions in the FY2018 Appropriations
The enacted FY2018 appropriation act for FSIS provided $1,056.8 million (Figure 1, Table 1). Compared to FY2011, the FY2018 appropriation for FSIS is nearly $50 million higher.
Of the total amount appropriated to FSIS, the FY2018 act requires that $7.5 million remain available until expended for public health veterinarian recruitment and retention incentives. As in previous years, congressional appropriators direct FSIS to employ no fewer than 148 FTEs during FY2018 that would be dedicated solely to inspections and enforcement related to the Humane Methods of Slaughter Act (7 U.S.C. 1901 et seq.).
The FY2018 appropriation for FSIS is divided into various discretionary subaccounts: federal food safety inspection ($943.8 million), state food safety inspection ($61.7 million), international inspections ($16.8 million), and the Public Health Data Communications Infrastructure System ($34.6 million).26
Language in the enacted FSIS appropriation, along with other statements in the House and the Senate committee reports, also addresses a number of concerns. These include certain animal welfare issues, catfish inspection and grading, and restrictions on horse slaughter and imports of chicken from China. Table 3 and Table 4 provide a summary of selected provisions.
Regarding catfish inspection, the act requires FSIS to issue equivalence determinations for all countries wishing to continue exporting Siluriformes (catfish) to the United States within 180 days of enactment.27 The explanatory statement on the enacted FY2018 act (P.L. 115-141) provides that $8 million be made available to fully implement Siluriformes fish and fish product inspection.28
Also, as in previous years, the enacted FY2018 appropriations act prohibits FSIS from using funds to inspect horse slaughter facilities or to make use of voluntary inspection fees.29 Horses are an amenable species under the Federal Meat Inspection Act, and FSIS is responsible for horse slaughter inspection if the horsemeat is for human consumption.
Finally, the enacted FY2018 appropriations act also prohibits the purchase of processed (cooked) poultry meat imported from China for use in the National School Lunch Program, the Child and Adult Food Care Program, the Summer Food Service Program for Children, and the School Breakfast Program.30 This provision has been added to enacted appropriations bills since FY2015 given continued concerns about China's poor food safety record. The enacted FY2018 act also contains a provision prohibiting the use of funds to finalize USDA's proposed rule regarding China's eligibility to export its poultry products to the United States unless USDA takes additional steps to ensure the equivalence of China's poultry slaughter inspection system, among other requirements.31
Congressional appropriations are augmented by existing user fees (overtime/holiday inspection services) and existing trust fund accounts (voluntary inspection services). In recent years, user fees and other available funds have generated between $180 million and $250 million per year (Figure 2), most of which is comprised of existing FSIS user fees.32
Selected Provisions in the FY2019 Appropriations
The House-reported bill and the Senate-passed bill would appropriate $1,049.3 million for FY2019 (Table 1), roughly $17 million more than the Administration's request.33
The Administration is proposing new user fees that would require establishments and official plants to pay fees to cover the costs of federal, state, and international inspection programs for meat, poultry, and eggs. The proposed fee would generate an estimated $660 million.34 Congressional appropriators have not accepted similar proposals from previous Administrations.
Language in the House and Senate FY2019 FSIS appropriation, along with other statements in the House and the Senate committee reports, address other food safety issues under FSIS jurisdiction (Table 3) that are similar to those enacted in the FY2018 appropriations act. These include provisions that address animal welfare, catfish inspection and grading, and restrictions on horse slaughter and imports of chicken from China. The House and the Senate committee bills and reports also contain other FSIS provisions. Table 3 and Table 4 provide a summary of selected provisions proposed for FY2019 and compare them to those in the FY2018 appropriations act.
A debate on which agency—FSIS or FDA—has regulatory jurisdiction over cell-cultured meat surfaced in early 2018.35 Both agencies have released public statements claiming oversight of the new technology. The House-reported bill includes a general provision that requires USDA "for fiscal year 2018 and hereafter" to regulate cell-cultured products made from cells of amenable species of livestock, as defined in the Federal Meat Inspection Act (21 U.S.C. §§601 et seq.), or poultry, as defined in the Poultry Products Inspection Act (21 U.S.C. §§451 et seq.).36 Elsewhere in the House committee report, however, appropriators acknowledge that the federal jurisdiction of cell-cultured products remains to be determined.37 In November 2018, a joint statement from FDA and FDA announced that both agencies "should jointly oversee the production of cell-cultured food products derived from livestock and poultry."
Provisions in Bill Text and Report Language
Table 3 compares selected policy provisions that have been identified in the Agriculture Programs (Title I), the Related Agencies and Food and Drug Administration (Title VI), and General Provisions (Title VII) titles of the FY2018 Agriculture appropriations act and the FY2019 Agriculture appropriations bills related to federal food safety activities at both FDA and FSIS. Many of these provisions have been included in past years' appropriations acts.
Table 4 compares selected policy provisions in the FY2018 and the FY2019 Agriculture appropriations report language or explanatory statements related to federal food safety activities at both FDA and FSIS. Many of these provisions have also been included in past years' appropriations acts.
Table 3. Comparison of Selected Appropriations Provisions in the Enacted FY2018 Appropriations Act and House and Senate Bill Text for FY2019
|
FY2018
|
FY2019
|
|
Enacted (P.L. 115-141)
|
House-Reported (H.R. 5961)
|
Senate-Passed (H.R. 6147)
|
|
FDA Provisions
|
|
|
|
FSMA Regulations
|
|
|
|
Restricts FDA funds from being used to implement FSMA regulation of the production, distribution, sale, or receipt of dried spent grains from the alcoholic beverage production process (§735). (See S.Rept. 115-131; H.Rept. 115-232.)
|
Similar to P.L. 115-141 (§733).
|
Similar to P.L. 115-141 (§735).
|
|
No comparable provision.
|
Restricts FDA funds from being used to enforce its final produce standards with respect to regulating the production, distribution, sale, or receipt of grape varietals that are grown, harvested, and used solely for wine (§755).
|
Similar to House provision (§753).
|
|
GE Salmon
|
|
|
|
Prohibits the introduction into interstate commerce of foods that contains genetically engineered salmon until labeling guidelines are finalized (§770).
|
Establishes disclosure requirements related to GE salmon or other finfish in accordance with USDA rules (§766).
|
Similar to P.L. 115-141 (§740).
|
|
Fish Advisories
|
|
|
|
No comparable provision.
|
No comparable provision.
|
Directs FDA to provide certain advice regarding its notice (along with the U.S. Environmental Protection agency) about eating fish (82 Federal Register 6571) (§764).
|
|
Directed Funding Allocations
|
|
|
|
Of available funds, requires $1.5 million for HHS's Office of Inspector General for oversight of FDA programs and operations (P.L. 115-141, FDA general text).
|
Similar to P.L. 115-141 (FDA general text).
|
Similar to P.L. 115-141 (FDA general text). See also S.Rept. 115-259.
|
|
Of available funds, requires $1.5 million for FDA and USDA coordinated public education activities on the safety and benefits of crop biotechnology and food and animal feed ingredients derived from biotechnology (P.L. 115-141, Explanatory Notes, p. 85).
|
Similar to P.L. 115-141 (FDA general text), but increases amount to $3 million.
|
No comparable provision.
|
|
No comparable provision.
|
No comparable provision.
|
Of available funds, requires $15 million be used for inspections of imported seafood (FDA general text).
|
|
Selected Policy Riders
|
|
|
|
Directs FDA not to deem partially hydrogenated oils as unsafe or unadulterated (§738).
|
Similar to P.L. 115-141 (§740).
|
Similar to P.L. 115-141 (§736).
|
|
Restricts FDA funds from being used to develop, issue, promote, or advance sodium regulations (§764).
|
Similar to P.L. 115-141 (§752).
|
Similar to P.L. 115-141 (§750).
|
|
Other Selected General Provisions
|
|
|
|
Directs FDA, and other federal agencies, to submit to appropriators a detailed spending plan by program, project, and activity for all funds made available (P.L. 115-141, §723).
|
Similar to P.L. 115-141 (§722).
|
Similar to P.L. 115-141 (§723).
|
|
FSIS Provisions
|
|
|
|
Federal Meat, Poultry, and Egg Inspection
|
|
|
|
Directs FSIS to employ no fewer than 148 FTEs during FY2018 dedicated solely to inspections and enforcement related to the Humane Methods of Slaughter Act (P.L. 115-141, FSIS general text).
|
Similar to P.L. 115-141 (FDA general text).
|
Similar to P.L. 115-141 (FSIS general text).
|
|
Prohibits imports from China of raw or processed poultry products for a number of school lunch and food programs (§756). Restricts the use of funds to finalize USDA's proposed rule regarding China's eligibility to export its poultry products to the United States (§760)
|
Includes similar language to P.L. 115-141 (§748 and §751).
|
No comparable language.
|
|
Prohibits funds from being used for horse slaughter and inspection (§782).
|
No comparable language.
|
Similar to P.L. 115-141 (§758).
|
|
Catfish Inspection
|
|
|
|
Requires that FSIS issue equivalence determinations for all countries wishing to continue exporting Siluriformes to the United States (P.L. 115-141, p. 74).
|
No comparable language.
|
Directs FSIS to continue implementing regulations on catfish inspection and grading (FSIS general text).
|
|
Country Audits
|
|
|
|
Directs USDA to conduct country audits related to, among other things, animal disease control and surveillance, lab capabilities, preparedness, and response (§742).
|
No comparable language.
|
No comparable language.
|
|
Products from Cells of Amenable Species
|
|
|
|
No comparable language.
|
Directs FSIS to regulate products made from cells of amenable species of livestock (§736).
|
No comparable language.
|
|
Veterinarian Recruitment and Retention
|
|
|
|
Of available funds, requires $7.5 million remain available until expended for public health veterinarian recruitment and retention (P.L. 115-141, p. 74).
|
No comparable language.
|
No comparable language.
|
Table 4. Selected Appropriations Provisions in Report Language, FY2018 and FY2019
|
FY2018
|
FY2019
|
|
Accompanying Report Language
(S.Rept. 115-131; H.Rept. 115-232; and Explanatory Statement)
|
House Committee Report (H.Rept. 115-706)
|
Senate Committee Report (S.Rept. 115-259)
|
|
FDA Provisions
|
|
|
|
FSMA's Regulatory Requirements/Assessment
|
|
|
|
Directs FDA to distinguish between grape varieties for wine production and grapes consumed raw (S, H).
|
No comparable provision, but see H.R. 5961, §755.
|
No comparable provision, but see H.R. 6174, §754.
|
|
Directs FDA to continue its progress in improving federal oversight and monitoring of state inspection programs, reviewing and strengthening internal directives and processes, and identifying new methods to improve oversight capabilities, including modernizing IT systems and infrastructure (H).
|
No comparable provision.
|
No comparable provision.
|
|
Encourages FDA to fully use the Centers for Food Safety and Nutrition Centers of Excellence to support research of FSMA activities (S).
|
No comparable provision.
|
Similar to language in S.Rept. 115-131.
|
|
Expresses concerns about regulations on byproducts of cotton ginning and cottonseed for animal feed use (S).
|
No comparable provision.
|
Similar to language in S.Rept. 115-131.
|
|
Provides sufficient monies to fund the FDA's Food Contact Notification Program but does not include proposed user fees (H). Recommendations do not include proposed user fees (S).
|
Provides sufficient funds for FDA's Food Contact Notification Program.
|
Recommends that proposed Food Contact Notification user fees not be included.
|
|
Directs FDA to further clarify compliance by small farms and urges FDA to communicate via guidance and technical assistance (S).
|
No comparable provision.
|
Similar to language in S.Rept. 115-131.
|
|
Imported Foods
|
|
|
|
Requests FDA provide a timeline of all activities associated with the investigation into the illnesses associated with pet food imports. Also requests FDA provide semi-annual reports on the status of the investigation until the issue has been resolved (H).
|
Requests that FDA provide a timeline of all activities associated with its investigation into pet illnesses associated imported pet food.
|
Recommends increases of $2.8 million for food import safety activities (as part of general increases for food safety activities).
|
|
Maintains FY2017 funding of $7.5 million for FDA's Office of Global Regulatory Operations and Policy to enhance the compliance and verification of foreign high-risk manufacturers and exporters of food and other products (H, S).
|
Directs FDA to spend $7.5 million of FY2018 appropriations for foreign high-risk inspections.
|
Directs FDA to provide an update on efforts to address foreign high-risk inspections.
|
|
Directs FDA to submit a report on the potential for implementing pilot programs allowing for public-private partnerships at high-volume ports of entry (H).
|
No comparable provision.
|
No comparable provision.
|
|
Directs FDA to work with local governments at high-volume ports of entry to reduce the risk of food-borne illnesses and improve local capacity (H). States it is the sense of the committee that FDA, USDA, and Customs and Border Protection should reassess staffing models to ensure available workforce at U.S. ports of entry (H).
|
Directs FDA to work with local governments at high-volume ports of entry to reduce the risk of food-borne illnesses and report back to the committee.
|
No comparable provision.
|
|
Working with States and Other Partners
|
|
|
|
Recommends FDA consider suggestions from its public hearing (''Strategic Partnerships to Enhance the Safety of Imported Foods') regarding the use of private-sector third-party auditors to help implement FSMA (S).
Encourages FDA to work in partnership with existing government food safety programs through memorandums of understanding (MOUs) to verify compliance with FSMA to rules once they are finalized as a way to eliminate duplication and provides $5 million for the Food Safety Outreach Program under the National Institute of Food and Agriculture (serving as the sole agency providing food safety training, education, outreach, and technical assistance at the farm level) (H).
Directs FDA to work with any state that designates a new state implementing agency to ensure it continues to receive funding under existing cooperative agreements (S).
|
Encourages FDA to enter into MOUs with government food safety programs.
Provides $5 million for USDA's Food Safety Outreach Program.
Directs FDA to continue its FSMA education and outreach to domestic and foreign producers, processors, and manufacturers.
Urges FDA to provide sufficient resources to state education/inspection programs.
|
Recommends increases of $7.2 million for FSMA cooperative agreements and $5 million to address food safety outbreaks (as part of general increases for food safety activities).
Directs FDA to work with any state that designates a new implementing agency to ensure it receives funding under existing cooperative agreements.
|
|
Seafood Safety
|
|
|
|
Provides $2.8 million to help develop lab methods to detect evidence of seafood decomposition (E, H).
|
No comparable provision.
|
No comparable provision.
|
|
No comparable provision.
|
No comparable provision.
|
Urges FDA to complete single lab validation and testing for detecting brevetoxins associated with neurotoxic shellfish poisoning in molluscan shellfish.
|
|
No comparable provision.
|
No comparable provision.
|
Of monies available for the National Antimicrobial Resistance Monitoring System, directs FDA to use $0.5 million to test antibiotic resistance in imported seafood.
|
|
Encourages FDA to increase funding for research into Vibrio illnesses associated with the consumption of raw molluscan shellfish (S).
|
No comparable provision.
|
Similar to language in S.Rept. 115-131.
|
|
Directs FDA to review its seafood advisory for pregnant and nursing women (S, H).
|
Directs FDA to post nutrient values of fish consumption during pregnancy on its website.
|
Directs FDA to reissue its final "Advice About Eating Fish'' (82 Federal Register 6571).
|
|
Standards of Identity; Food Fraud/Defense
|
|
|
|
Directs FDA to develop a dairy-specific standard of identity and guidance in accordance with the Dairy Pride Act, H.R. 778/S. 130 (H).
|
Directs FDA to develop a standard of identity for dairy products and issue guidance on how to implement and enforce the standard.
|
No comparable provision.
|
|
Directs FDA to monitor olive oil import fraud (S, H), update its 2014 olive oil survey (H), and respond to a 2012 citizen petition requesting a standard of identity for olive oil and olive pomace oil (S).
|
Directs FDA to establish a separate U.S. standard of identity for different grades of olive oil.
|
Directs FDA to establish a standard of identity for different grades of olive oil and olive-pomace oils.
|
|
Urges FDA to engage in further collaborative dialogue with stakeholders regarding its final rule ''Mitigation Strategies to Protect Food Against Intentional Adulteration'' (81 Federal Register 34165) (E).
|
No comparable provision.
|
Encourages FDA to work with businesses to identify food defense practices that effectively protect public health.
|
|
Directs FDA to define natural and establish a uniform national labeling standard (H).
|
No comparable provision.
|
No comparable provision.
|
|
Of the amount provided for CFSAN, requires an increase of $3 million for the Office of Nutrition and Food Labeling to prioritize efforts regarding standards of identity and related product labeling (H).
|
Similar to language in H.Rept. 115-232.
|
No comparable provision.
|
|
Directs FDA to address concerns about misleading maple syrup and product marketing (S).
|
No comparable provision.
|
Similar to language in S.Rept. 115-131.
|
|
Other Selected Provisions
|
|
|
|
Encourages the National Academies of Sciences, Engineering, and Medicine to conduct a study on new technologies to promote microbiological food safety and prevent foodborne illnesses (H).
|
No comparable provision.
|
No comparable provision.
|
|
Directs FDA to consider all risk assessments before finalizing the guidance and policy on Listeria Monocytogenes to ensure it is risk-based and reflects good and achievable industry practice (H).
|
Similar to language in H.Rept. 115-232.
|
Urges FDA to complete a comprehensive risk assessment before changing its guidance regarding Listeria Monocytogenes in ready-to-eat foods.
|
|
No comparable provision.
|
Provides $3 million for USDA and FDA to educate the public on the safety and benefits of crop biotechnology and food and animal feed ingredients derived from biotechnology.
|
No comparable provision.
|
|
Encourages FDA and USDA to provide outreach and guidance to food manufacturers and retailers on food date labeling (H).
|
Similar to language in H.Rept. 115-232.
|
No comparable provision.
|
|
Directs FDA to comply with Title 31 of the U.S. Code regarding the development of its organizational priority goals and outcomes (e.g., performance outcome and output measures, and also efficiency and customer service measures) (H).
|
Similar to language in H.Rept. 115-232. Also directs FDA to provide public information on the link between FSMA activities and performance measures, especially public health outcome measures.
|
No comparable provision.
|
|
No comparable provision.
|
No comparable provision.
|
Urges FDA to dedicate extra personnel to speed the review and approval process regarding animal feed ingredients.
|
|
Maple Syrup Labeling
|
|
|
|
Directs FDA to report to the committee on its efforts to implement regulations and provide clarity to the maple syrup and honey industries on the labeling of the sugar content of their packaged products (S).
|
No comparable provision, but see H.R. 5961, §764.
|
Directs FDA to work with maple syrup and honey sectors on labeling for single-ingredient products where sugar is naturally occurring (not added). See also H.R. 6174, §768.
|
|
Selected Policy Riders, Other Guidance
|
|
|
|
Directs FDA to extend the compliance date for manufacturers for the Nutrition Facts Label Final Rule and the Serving Size Final Rule and finalize these regulations before July 26, 2018 (E). Directs FDA to issue guidance on pending dietary fiber ingredients (E).
Addresses menu labeling requirements and nutrition facts labeling, including guidance regarding the definition of dietary fiber and labeling of added sugars (S, H).
|
Urges FDA to reduce the burden on and add flexibility for businesses under the menu labeling rule.
Directs FDA to allow three years for industry compliance after final guidance is issued regarding dietary fiber sources.
|
Includes language regarding dietary fiber similar to language in S.Rept. 115-131.
Directs FDA to report on activities and resources spent on nutrition-related activities of CFSAN and associated field offices.
|
|
FSIS Provisions
|
|
|
|
Humane Methods of Slaughter
|
|
|
|
Directs FSIS to provide an annual report on the implementation of objective scoring methods undertaken to enforce the Human Methods of Slaughter Act (S, H).
|
Directs FSIS to ensure that inspectors comply with the Humane Methods of Slaughter Act.
|
Directs FSIS to ensure that inspectors comply with the Humane Methods of Slaughter Act. Requires annual reporting.
|
|
Siluriformes (or Catfish) Inspection
|
|
|
|
Provides $8 million to fully implement Siluriformes fish and fish product inspection (E). Directs FSIS to complete equivalence determinations for all countries wishing to continue exporting Siluriformes to the United States. In addition, directs FSIS to complete equivalence determinations on a country-by-country basis based on volume of catfish exports to the United States (S, H).
|
Restates FSIS is expected to comply with the FY2018 enacted appropriation in determining equivalency for countries wishing to export catfish to the United States.
|
No comparable language.
|
|
Cultured Meat
|
|
|
|
No comparable language.
|
Acknowledges regulatory responsibility is still being determined for cell-cultured meat. (See "Minority views of the Hon. Nita Lowey and the Hon. Sanford D. Bishop, Jr."). See also H.R. 5961, §736.
|
No comparable language.
|
|
Breed Claim Standards
|
|
|
|
Direct FSIS to (1) evaluate current processes for the labeling of 100% pure breed claim standards for livestock products and (2) identify instances of conflicting 100% pure breed claim standards for livestock (S).
|
No comparable language.
|
No comparable language.
|
|
Game Birds
|
|
|
|
Directs FSIS to provide a report on the impact of defining games birds (e.g., quail) as amendable in federal code regarding inspection (S).
|
No comparable language.
|
No comparable language.
|
Source: CRS.
Notes: Provisions in the enacted FY2018 Explanatory Statement are cited as (E) and available at U.S. Congress, House Committee on Appropriations, Committee Print on H.R. 1625/P.L. 151-141, Book 1—Consolidated Appropriations Act, 2018, 115th Cong., 2nd sess., 29-456. Provisions listed in the House report (H.Rept. 115-232) are cited as (H). Provisions listed in the Senate report (S.Rept. 115-131) are cited as (S).