The Food and Drug Administration (FDA) Budget: Fact Sheet
Changes from July 10, 2017 to September 12, 2018
This page shows textual changes in the document between the two versions indicated in the dates above. Textual matter removed in the later version is indicated with red strikethrough and textual matter added in the later version is indicated with blue.
The Food and Drug Administration (FDA) Budget: Fact Sheet
Contents
- FDA Overview
- Funding Sources
- FDA
RecentFunding History andthe FY2017 OmnibusFY2018 Appropriations
Summary
The Food and Drug Administration (FDA) regulates the safety of foods (including dietary supplements), cosmetics, and radiation-emitting products; the safety and effectiveness of drugs, biologics (e.g., vaccines), and medical devices; and public health aspects of tobacco products. Seven centers within FDA represent the broad program areas for which the agency has responsibility:FDA is organized into various offices and centers that carry out the agency's regulatory responsibilities. The Office of the Commissioner and four other program area offices oversee the core functions of the agency: the Office of Medical Products and Tobacco, the Office of Foods and Veterinary Medicine, the Office of Global Regulatory Operations and Policy, and the Office of Operations. The Office of Medical Products and Tobacco includes the Center for Biologics Evaluation and Research (CBER), the Center for Devices and Radiological Health (CDRH), the Center for Drug Evaluation and Research (CDER), and the Center for Tobacco Products (CTP), while the Office of Foods and Veterinary Medicine includes the Center for Food Safety and Applied Nutrition (CFSAN), and the Center for Veterinary Medicine (CVM), the. The National Center for Toxicological Research (NCTR), and the Center for Tobacco Products (CTP). Several other offices have agency-wide responsibilities.
FDA's budget has two funding streams: annual appropriations (i.e., discretionary budget authority, or BA) and industry user fees. In FDA's annual appropriation, Congress sets both the total amount of appropriated funds and the amount of user fees that the agency is authorized to collect and obligate for that fiscal year.
Between FY2012 and FY2017, FDA's FY2014 and FY2018, FDA's enacted total program level increased from $3.8324.387 billion to $4.7455.269 billion. AlthoughOver this time period, congressionally appropriated funding increased by 11% over that time period,12%, and user fee revenue increased more than 47by 31%. The Administration's FY2018FY2019 budget request was for a total program level of $5.112772 billion, an increase of $367503 million (+89%) over the FY2017FY2018-enacted amount ($4.7455.269 billion). This report will be updated with information on FDA funding for FY2018FY2019 once legislative action on appropriations for the new fiscal year is completed.
FDA Overview
The Food and Drug Administration (FDA) regulates the safety of foods (including dietary supplements), cosmetics, and radiation-emitting products; the safety and effectiveness of drugs, biologics (e.g., vaccines), and medical devices; and public health aspects of tobacco products.1 Although FDA has been a part of the Department of Health and Human Services (HHS) since 1940, the Committees on Appropriations do not consider FDA within the rest of HHS under their Subcommittees on Labor, Health and Human Services, and Education, and Related Agencies. Jurisdiction over FDA's budget remains with the Subcommittees on Agriculture, Rural Development, Food and Drug Administration, and Related Agencies, reflecting FDA's beginnings as part of the Department of Agriculture.
FDA Centers FDA's organization consists of various offices and centers that carry out the agency's regulatory responsibilities. The Office of the Commissioner and four other program area offices oversee the core functions of the agency: the Office of Medical Products and Tobacco, the Office of Foods and Veterinary Medicine, the Office of Global Regulatory Operations and Policy, and the Office of Operations. The Office of Medical Products and Tobacco includes the Center for Biologics Evaluation and Research (CBER), the Center for Devices and Radiological Health (CDRH) , the Center for Drug Evaluation and Research (CDER) Center for Food Safety and Applied Nutrition (CFSAN) Center for Tobacco Products (CTP) , and the Center for Tobacco Products (CTP), while the Office of Foods and Veterinary Medicine includes the Center for Food Safety and Applied Nutrition (CFSAN) and the Center for Veterinary Medicine (CVM) . The National Center for Toxicological Research (NCTR) is housed within the Office of the Commissioner.2
The agency's budget—as presented in the Justifications of Estimates for Appropriations Committees (referred to as "Congressional Justifications," or CJs) and the materials of the Committees on Appropriations—is organized by program area. Consistent with these budget documents, Table 1 displays funding for FY2014 through FY2018, as well as the Administration's FY2019 request, by program area (e.g., Foods, Human Drugs), which includes funding for the responsible FDA center (e.g., CFSAN, CDER) and the portion of funding for the FDA-wide Office of Regulatory Affairs (ORA) that is committed to that program area.3 |
Seven centers within FDA represent the broad program areas for which the agency has responsibility, along with various other offices that have agency-wide responsibilities. Table 1 is organized in a format consistent with the Administration's budget request as presented in the FDA Congressional Justification, as well as with the materials of the Committees on Appropriations—each program area includes funding (FY2013-FY2017, as well as the Administration's FY2018 request) designated for the responsible FDA center (e.g., CDER or CFSAN) and the portion of funding for the FDA-wide Office of Regulatory Affairs that is committed to that program area.
Funding Sources
FDA's total program level, the amount that FDA can spend, is composed of direct appropriations (also referred to as budget authority) and user fees.2 In FDA's annual appropriation, Congress sets both the amount of appropriated fundsdiscretionary appropriations from two different sources. First, FDA is appropriated funding out of the Treasury's General Fund. (This is the usual source of funding for discretionary appropriations, and, in keeping with the conventions used in FDA budget documents, is referred to in this report as budget authority.)4 Second, FDA also is allowed to collect and obligate user fees.5 FDA's annual appropriation sets both the amount of budget authority and the amount of user fees that the agency is authorized to collect and obligate for that fiscal year. Appropriated fundsThe budget authority appropriations are largely for the Salaries and Expenses account, with a much smaller amount for the Buildings and Facilities account. The, which is used for any changes to or purchase of fixed equipment and facilities used by FDA.6 The appropriations of the several different user fees contribute only to the Salaries and Expenses account.
The largest and oldest FDA user fee that is linked to a specific program was first authorized by the Prescription Drug User Fee Act (PDUFA, P.L. 102-571) in 1992. After PDUFA, Congress added user fee authorities regarding medical devices, animal drugs, animal generic drugs, tobacco products, priority review, food reinspection, food recall, voluntary qualified food importer, generic drugs, biosimilars, and, most recently, outsourcing facilities (related to drug compounding), and some wholesale distributors and third-party logistics providers (related to pharmaceutical supply chain security).3 Each of the medical product fee authorities requires reauthorization every five years. Several indefinite authorities apply to fees for mammography inspection, color additive certification, export certification, priority review vouchers, food and feed recall, food inspection, voluntary qualified food importers, third-party auditor, and outsourcing facilities.
FDA Recent Funding History and the FY2017 Omnibus
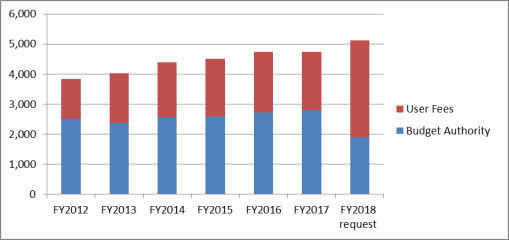
Between FY2012 and FY2017, FDA's total program level increased from $3.832 billion to $4.745 billion. Although congressionally appropriated funding increased by 11% over that time period, user fee revenue increased more than 47%. In FY2017, user fees account for 41% of FDA's total program level. Under the Trump Administration's FY2018 request, user fees would account for 63% of the FDA's total program level.4
(in millions of dollars) |
 |
|
The Administration's FY2018 request includes a total program level of $5.112 billion, an increase of $367 million (+8%) over the FY2017 enacted amount.5 The FY2018 request proposes $1.888 billion in direct appropriations—a decrease of $903 million (-32%) from the FY2017 enacted amount. The FY2018 request includes in the direct appropriation $60 million from the FDA Innovation Account, which was established by Section 1002 of the 21st Century Cures Act (P.L. 114-255).6
The 21st Century Cures Act (P.L. 114-255), signed into law in December 2016, made several changes to the drug and device approval pathways at FDA to support innovation and accelerate development and review of certain medical products (e.g., combination products, antimicrobials, drugs for rare disease, and regenerative therapies). To fund these activities, the Cures Act established an FDA Innovation Account to which a total of $500 million is authorized to be transferred over a nine-year period (FY2017-FY2025).7 The law specified that amounts in the account are not available until appropriated in subsequent appropriations acts and that once made available, these amounts are available until expended. The amounts subsequently appropriated (i.e., the budget authority and the resulting outlays) for FY2017 through FY2025, up to the amounts transferred, are to be subtracted from any cost estimates provided for purposes of budget controls. Effectively, the appropriations from the account will not be counted against any spending limits, such as the statutory discretionary spending limits; that is, the amounts appropriated from the account will be considered outside those limits for FY2017 through FY2025.
(in millions of dollars) Source: Figure created by CRS using the FY1992 through FY2019 FDA CJs and the Consolidated Appropriations Act of 2018 (P.L. 115-141). Notes: These amounts have not been adjusted for inflation. The purpose of this figure is to show how FDA's spending has changed over time to include a greater proportion from user fees compared to budget authority. With the exception of FY2018, which reflects the enacted appropriation, the amounts used in this figure are from the "Actuals" columns in the FDA CJs, which, according to the FY2005 CJ, reflect FDA's actual spending rather than what was provided in the enacted appropriation. PDUFA= Prescription Drug User Fee Act; MDUFMA= Medical Device User Fee and Modernization Act; ADUFA= Animal Drug User Fee Act; AGDUFA= Animal Generic Drug User Fee Act; TCA= The Family Smoking Prevention and Tobacco Control Act; FSMA= Food Safety Modernization Act; BsUFA= Biosimilar User Fee Act; GDUFA= Generic Drug User Fee Amendments.For user fees, the FY2018 request proposes $3.223 billion in fees—an increase of $1.269 billion or 65% over the FY2017, while the indefinite or permanent authorities do not require reauthorization. Table A-1 presents the list of user fees that contribute to FDA's budget, sorted by the dollar amount they contribute to the agency's FY2018 budget. The table also includes the authorizing legislation for each current user fee, specifies whether the user fee program is indefinite or requires reauthorization, and provides the most recent reauthorization, if applicable.
Between FY2014 and FY2018, FDA's enacted total program level increased from $4.387 billion to $5.269 billion (see Table 1). Over that time period, congressionally appropriated funding increased by 12% while user fee revenue increased more than 31%.
The Administration's FY2019 request includes a total program level of $5.772 billion, an increase of $503 million (+10%) over the FY2018-enacted amount. The FY2019 request proposes $3.254 billion in budget authority—an increase of $382 million (+13%) over the FY2018-enacted amount. This amount includes $70 million for the FDA Innovation Account, as specified in the 21st Century Cures Act. Table 1 includes the FDA Innovation Account money in the total budget authority and program level amounts, consistent with the budget display conventions used in the FDA CJs.
The FY2019 request proposes $2.519 billion in fees—an increase of $122 million (5%) over the FY2018 enacted amount—to be collected through authorized programs to support specified agency activities regarding prescription drugs, medical devices, animal drugs, animal generic drugs, tobacco products, generic human drugs, biosimilars, mammography quality, color certification, export certification, food reinspection, food recall, the voluntary qualified importer program, outsourcing facilities, priority review vouchers, and third-party auditors. In addition to the $
3.2232.519 billion in user fees from currently authorized programs, the Administration requests for FY2018 an additional $4.28 million in as yet unauthorized fees to support export certification activities. With those proposed fees, the Administration's total user fee request comes to $3.228 billion, bringing the program level request to $5.116 billion.
Not included in any of these totals is the $10 million (to the Salaries and Expenses account) provided by Section 752 of the FY2017 enacted appropriation for FDA to "prevent, prepare for, and respond to emerging health threats, including the Ebola and Zika viruses, domestically and internationally and to develop necessary medical countermeasures and vaccines, including the review, regulation, and post market surveillance of vaccines and therapies, and for related administrative activities ... to remain available until expended."
FY2019, in as yet unauthorized fees, an additional $4.28 million to support export certification activities and $22 million to support over-the-counter (OTC) drug monograph activities.8
Consistent with the Administration and congressional budget display conventions, Table 1 displays, by program area, the budget authority (direct appropriations), user fees, and total program levels for FDA from FY2014 through FY2018 and the FY2019 request. The human drugs program comprises the largest portion of FDA's budget (2831% in FY2017FY2018), followed by the foods program (2220% in FY2017FY2018), and the tobacco program (13% in FY2017). Table 1 displays, by program area, the budget authority (direct appropriations), user fees, and total program levels for FDA in previous years.
|
Program Area |
FY2012 | FY2013 |
FY2014 |
FY2015 |
FY2016 |
FY2017 |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Foods |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Human drugs |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Biologics |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Animal drugs and feeds |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Devices and radiological health |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Tobacco products |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Toxicological research (NCTR) |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
Fees |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Headquarters/ Commissioner's Office
|
|
|
|
GSA rent |
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
BA |
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fees
|
|
Fees |
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GSA rent |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
Fees |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fees
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
Fees |
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
Priority review voucher |
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
Buildings & Facilities |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees
|
|
|
|
|
BA |
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Priority review voucher |
|
|
Total Budget Authority |
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
BA
|
|
Total User Fees |
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Buildings & Facilities |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
BA |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fees |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Total Budget Authority |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Total User Fees |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Total Program Level |
|
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sources: FY2012 amounts are from the FY2013 Sequestration Operating Plan. FY2013 and FY2014 amounts are from the FDA FY2014 Operating Plan. FY2013 figures reflect sequestration. FY2014 amounts are from the FDA FY2014 Operating Plan. The FY2015 and FY2016 amounts are from the FDA FY2016 Operating Plan. The FY2017 amounts are from the 2017 Consolidated Appropriations Act (P.L. 115-31) and its Explanatory Statement (for H.R. 244 in the May 3, 2017, Congressional Record), as well as the 2017 Further Continuing and Security Assistance Appropriations Act (P.L. 114-254). The FY2018 request amounts are from the FY2018 FDA Justification of Estimates for Appropriations Committees.
Notes: Individual amounts may not add to subtotals or totals due to rounding. Consistent with the Administration and congressional committee formats, each program area includes funding designated for the responsible FDA center (e.g., the Center for Drug Evaluation and Research or the Center for Food Safety and Applied Nutrition) and the portion budgeted for agency-wide Office of Regulatory Affairs in that area.
a. P.L. 114-113 (for FY2016) and P.L. 115-31 (for FY2017) required that $1.5 million of the budget authority provided for "other activities" (e.g., Office of the Commissioner) be transferred to the HHS Office of Inspector General for FDA oversight.
b.
Other rent and rent-related activities include White Oak consolidation.
c.
The FY2014-FY2017 amounts reflect the color certification fees authorized by the Color Additive Amendments of 1960 (P.L. 86-618) and export certification for medical products authorized by the FDA Export Reform and Enhancement Act of 1996 (P.L. 104-134). The Food Safety Modernization Act (FSMA) of 2011 (P.L. 111-353) authorized FDA to collect export certification fees also for food. Note that the Appropriations Committees have not included funding for the export certification fees authorized by FSMA.
d.
The FY2013 Sequestration Operating Plan notes food safety and drug safety items that had not been included in the program-level appropriations. Subsequent years' bills have not specified this distinct item.
e.
In December 2016, Congress passed a measure providing continuing appropriations for FY2017 through April 28, 2017 (P.L. 114-254). The law provided to the FDA an additional $20 million for FY2017, pursuant to the 21st Century Cures Act (P.L. 114-255), which establishes an FDA Innovation Account to help fund the agency's activities and programs authorized in Division A of the Cures Act (e.g., changes to the drug and device FDA approval pathways).
f.
Table VIII of P.L. 113-235 (for FY2015) provided an additional, one-time $25 million in direct appropriations to FDA for Ebola response and preparedness activities. Adding this $25 million to the FDA appropriations made in Title VI brought BA to $2.622 billion and the total program level to $4.525 billion for FY2015.
g.
For user fees in the Administration's FY2018 request, this column shows only the $3.223 billion in fees that have been authorized. The request included $4.2 million in proposed additional export certification fees. With these proposed user fees, the FY2018 request for user fees totals $3.228 billion yielding a total program level request of $5.116 billion.
h.
Section 752 provides an additional $10 million for FDA to "prevent, prepare for, and respond to emerging health threats...." Adding this $10 million to the FDA appropriations brings BA to $2.801 billion and the total program level to $4.755 billion for FY2017.
Author Contact Information
Footnotes
| 1. |
Several CRS reports have information on FDA authority and activities: CRS Report R41983, How FDA Approves Drugs and Regulates Their Safety and Effectiveness, and CRS Report R42130, FDA Regulation of Medical Devices. |
||
|
Beginning with the Prescription Drug User Fee Act (PDUFA, P.L. 102-571) in 1992, | |||
| 3. |
|
||
| 4. |
The human medical product user fee agreements for FY2018 through FY2022 have been negotiated and agreed to by FDA and the regulated industries and are reflected in authorizing legislation reported by the authorizing committees in the Senate and House (S. 934, H.R. 2430). The amounts in the Administration's FY2018 request do not reflect the user fee revenue agreed upon by FDA and industry. For additional information about the agreements, see CRS Report R44750 FDA Medical Product User Fee Reauthorization: In Brief, and CRS Report R44864 Prescription Drug User Fee Act (PDUFA): 2017 Reauthorization as PDUFA VI. |
||
| 5. |
The $4.745 billion includes $4.725 billion provided in the 2017 Consolidated Appropriations Act ("enacted appropriation;" P.L. 115-31), authorized user fees, and the $20 million provided in the second CR pursuant to 21st Century Cures. |
||
| 6. | For each of FY2017 through FY2025, the following amounts are authorized to be transferred to the FDA Innovation Account: $20 million in FY2017, $60 million in FY2018, $70 million in FY2019, $75 million in FY2020, $70 million in FY2021, $50 million in FY2022, $50 million in FY2023, $50 million in FY2024, and $55 million in FY2025. |