FDA Overview
The Food and Drug Administration (FDA) regulates the safety of foods (including dietary supplements), cosmetics, and radiation-emitting products; the safety and effectiveness of drugs, biologics (e.g., vaccines), and medical devices; and public health aspects of tobacco products.1 Although FDA has been a part of the Department of Health and Human Services (HHS) since 1940, the Committees on Appropriations do not consider FDA within the rest of HHS under their Subcommittees on Labor, Health and Human Services, and Education, and Related Agencies. Jurisdiction over FDA's budget remains with the Subcommittees on Agriculture, Rural Development, Food and Drug Administration, and Related Agencies, reflecting FDA's beginnings as part of the Department of Agriculture.
FDA's organization consists of various offices and centers that carry out the agency's regulatory responsibilities. The Office of the Commissioner and four other program area offices oversee the core functions of the agency: the Office of Medical Products and Tobacco, the Office of Foods and Veterinary Medicine, the Office of Global Regulatory Operations and Policy, and the Office of Operations. The Office of Medical Products and Tobacco includes the Center for Biologics Evaluation and Research (CBER), the Center for Devices and Radiological Health (CDRH), the Center for Drug Evaluation and Research (CDER), and the Center for Tobacco Products (CTP), while the Office of Foods and Veterinary Medicine includes the Center for Food Safety and Applied Nutrition (CFSAN) and the Center for Veterinary Medicine (CVM). The National Center for Toxicological Research (NCTR) is housed within the Office of the Commissioner.2
The agency's budget—as presented in the Justifications of Estimates for Appropriations Committees (referred to as "Congressional Justifications," or CJs) and the materials of the Committees on Appropriations—is organized by program area. Consistent with these budget documents, Table 1 displays funding for FY2014 through FY2018, as well as the Administration's FY2019 request, by program area (e.g., Foods, Human Drugs), which includes funding for the responsible FDA center (e.g., CFSAN, CDER) and the portion of funding for the FDA-wide Office of Regulatory Affairs (ORA) that is committed to that program area.3
Funding Sources
FDA's total program level, the amount that FDA can spend, is composed of discretionary appropriations from two different sources. First, FDA is appropriated funding out of the Treasury's General Fund. (This is the usual source of funding for discretionary appropriations, and, in keeping with the conventions used in FDA budget documents, is referred to in this report as budget authority.)4 Second, FDA also is allowed to collect and obligate user fees.5 FDA's annual appropriation sets both the amount of budget authority and the amount of user fees that the agency is authorized to collect and obligate for that fiscal year. The budget authority appropriations are largely for the Salaries and Expenses account, with a smaller amount for the Buildings and Facilities account, which is used for any changes to or purchase of fixed equipment and facilities used by FDA.6 The appropriations of the several different user fees contribute only to the Salaries and Expenses account.
The largest and oldest FDA user fee that is linked to a specific program was first authorized by the Prescription Drug User Fee Act (PDUFA, P.L. 102-571) in 1992. After PDUFA, Congress added user fee authorities regarding medical devices, animal drugs, animal generic drugs, tobacco products, priority review, food reinspection, food recall, voluntary qualified food importer, generic drugs, biosimilars, outsourcing facilities (related to drug compounding), and some wholesale distributors and third-party logistics providers (related to pharmaceutical supply chain security). Each of the medical product fee authorities requires reauthorization every five years, while the indefinite or permanent authorities do not require reauthorization. Table A-1 presents the list of user fees that contribute to FDA's budget, sorted by the dollar amount they contribute to the agency's FY2018 budget. The table also includes the authorizing legislation for each current user fee, specifies whether the user fee program is indefinite or requires reauthorization, and provides the most recent reauthorization, if applicable.
The 21st Century Cures Act (P.L. 114-255), signed into law in December 2016, made several changes to the drug and device approval pathways at FDA to support innovation and accelerate development and review of certain medical products (e.g., combination products, antimicrobials, drugs for rare disease, and regenerative therapies). To fund these activities, the Cures Act established an FDA Innovation Account to which a total of $500 million is authorized to be transferred over a nine-year period (FY2017-FY2025).7 The law specified that amounts in the account are not available until appropriated in subsequent appropriations acts and that once made available, these amounts are available until expended. The amounts subsequently appropriated (i.e., the budget authority and the resulting outlays) for FY2017 through FY2025, up to the amounts transferred, are to be subtracted from any cost estimates provided for purposes of budget controls. Effectively, the appropriations from the account will not be counted against any spending limits, such as the statutory discretionary spending limits; that is, the amounts appropriated from the account will be considered outside those limits for FY2017 through FY2025.
FDA Funding History and FY2018 Appropriations
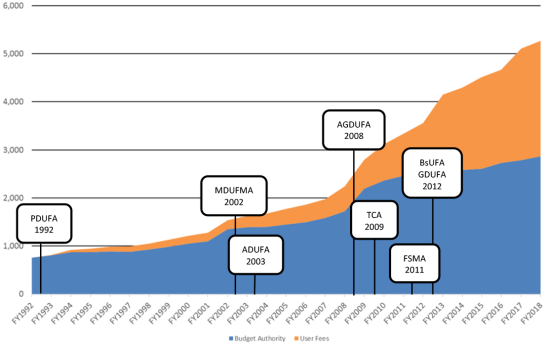
Since the enactment of PDUFA in 1992, FDA's spending from user fees has generally increased, both in absolute terms and as a share of FDA's total budget, accounting for 45% of the agency's FY2018 total program level (see Figure 1).
|
Figure 1. FDA Budget, by Source, FY1992-FY2018 (in millions of dollars) |
 |
|
Source: Figure created by CRS using the FY1992 through FY2019 FDA CJs and the Consolidated Appropriations Act of 2018 (P.L. 115-141). Notes: These amounts have not been adjusted for inflation. The purpose of this figure is to show how FDA's spending has changed over time to include a greater proportion from user fees compared to budget authority. With the exception of FY2018, which reflects the enacted appropriation, the amounts used in this figure are from the "Actuals" columns in the FDA CJs, which, according to the FY2005 CJ, reflect FDA's actual spending rather than what was provided in the enacted appropriation. PDUFA= Prescription Drug User Fee Act; MDUFMA= Medical Device User Fee and Modernization Act; ADUFA= Animal Drug User Fee Act; AGDUFA= Animal Generic Drug User Fee Act; TCA= The Family Smoking Prevention and Tobacco Control Act; FSMA= Food Safety Modernization Act; BsUFA= Biosimilar User Fee Act; GDUFA= Generic Drug User Fee Amendments. |
Between FY2014 and FY2018, FDA's enacted total program level increased from $4.387 billion to $5.269 billion (see Table 1). Over that time period, congressionally appropriated funding increased by 12% while user fee revenue increased more than 31%.
The Administration's FY2019 request includes a total program level of $5.772 billion, an increase of $503 million (+10%) over the FY2018-enacted amount. The FY2019 request proposes $3.254 billion in budget authority—an increase of $382 million (+13%) over the FY2018-enacted amount. This amount includes $70 million for the FDA Innovation Account, as specified in the 21st Century Cures Act. Table 1 includes the FDA Innovation Account money in the total budget authority and program level amounts, consistent with the budget display conventions used in the FDA CJs.
The FY2019 request proposes $2.519 billion in fees—an increase of $122 million (5%) over the FY2018 enacted amount—to be collected through authorized programs to support specified agency activities regarding prescription drugs, medical devices, animal drugs, animal generic drugs, tobacco products, generic human drugs, biosimilars, mammography quality, color certification, export certification, food reinspection, food recall, the voluntary qualified importer program, outsourcing facilities, priority review vouchers, and third-party auditors. In addition to the $2.519 billion in user fees from currently authorized programs, the Administration requests for FY2019, in as yet unauthorized fees, an additional $4.28 million to support export certification activities and $22 million to support over-the-counter (OTC) drug monograph activities.8
Consistent with the Administration and congressional budget display conventions, Table 1 displays, by program area, the budget authority (direct appropriations), user fees, and total program levels for FDA from FY2014 through FY2018 and the FY2019 request. The human drugs program comprises the largest portion of FDA's budget (31% in FY2018), followed by the foods program (20% in FY2018), and the tobacco program (12% in FY2018), which is funded solely by tobacco product user fees.
|
Program Area |
FY2014 Enacted |
FY2015 Enacted |
FY2016 Enacted |
FY2017 Enacted |
FY2018 Enacted |
FY2019 Request |
||||||
|
Foods |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
Human drugs |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
Biologics |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
Animal drugs and feeds |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
Devices and radiological health |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
Tobacco products |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
Toxicological research |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Headquarters/ Commissioner's Officea |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
GSA rent |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
Other rent, rent-related activitiesb |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
Export, color certificationc |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
Priority review voucher |
|
|
|
|
|
|
||||||
|
Fees |
|
|
|
|
|
|
||||||
|
FDA Innovation Account |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Buildings & Facilities |
|
|
|
|
|
|
||||||
|
BA |
|
|
|
|
|
|
||||||
|
Total Budget Authority |
|
|
|
|
|
|
||||||
|
Total User Fees |
|
|
|
|
|
|
||||||
|
Total Program Level |
|
|
|
|
|
|
Sources: The FY2014-FY2019 FDA CJs; the Consolidated Appropriations Act, 2014 (P.L. 113-76); the Consolidated and Further Continuing Appropriations Act, 2015 (P.L. 113-235); the Consolidated Appropriations Act, 2016 (P.L. 114-113); the Consolidated Appropriations Act, 2017 (P.L. 115-31); the 2017 Further Continuing and Security Assistance Appropriations Act (P.L. 114-254); the Consolidated Appropriations Act, 2018 (P.L. 115-141); and the accompanying explanatory statements.
Notes: Individual amounts may not add to subtotals or totals due to rounding. Consistent with the Administration and congressional committee formats, each program area includes funding designated for the responsible FDA center (e.g., the Center for Drug Evaluation and Research or the Center for Food Safety and Applied Nutrition) and the portion budgeted for agency-wide Office of Regulatory Affairs in that area.
a. The FY2015 through FY2018 amounts do not reflect the transfer of $1.5 million to the HHS Office of Inspector General for FDA oversight required in the enacted appropriation for those years. This transfer was not directed in the FY2014 appropriations law, and FDA proposed to discontinue this transfer in the FY2019 FDA Justification of Estimates for Appropriations Committees.
b. Other rent and rent-related activities include White Oak consolidation.
c. The FY2014-FY2018 amounts reflect the color certification fees authorized by the Color Additive Amendments of 1960 (P.L. 86-618) and export certification for medical products authorized by the FDA Export Reform and Enhancement Act of 1996 (P.L. 104-134). The Food Safety Modernization Act of 2011 (P.L. 111-353) authorized FDA to collect export certification fees also for food. Under current law, export certification fees paid to FDA are capped at $175 per certification. The FY2019 request proposes an increase in the cap of export certification fees from $175 to $600 per certification (i.e., an additional $4.28 million in proceeds from export certification fees).
d. The FDA funding table in the FY2016 Explanatory Statement (Congressional Record, vol. 161 no. 184—Book II, H9725-H9726, December 17, 2015) does not include the $7.686 million in priority review voucher user fees. However, according to the FDA funding table in the "FY 2016 enacted" column in the FY2017 Explanatory Statement (Congressional Record, vol. 163 no. 76—Book II, H3358-H3359, May 3, 2017), the $7.686 million was provided, which is consistent with the "FY 2016 Enacted" column in the FDA FY2017 CJ.
e. For user fees in the Administration's FY2019 request, this column shows only the $2.519 billion in fees that have been authorized. The Administration's request also includes $4.2 million in additional proposed export certification fees, as well as $22 million in proposed OTC drug monograph product fees. Including the proposed fees would bring the Administration's total requested user fee amount to $2.545 billion.
f. This total does not include the $25 million provided by Title VIII of P.L. 113-235 (for FY2015), to remain available until expended, to FDA for Ebola response and preparedness activities.
g. This total does not include the $10 million provided by Section 752 of P.L. 115-31 (for FY2017), to remain available until expended, for FDA to "prevent, prepare for, and respond to emerging health threats..."
h. This total does not include the $94 million provided by Section 778 of P.L. 115-141 (for FY2018), to remain available until expended, for FDA to expand efforts related to processing opioids and other articles imported through international mail facilities of the U.S. Postal Service. This total also does not include $7.6 million in one-time, no year funding for Hurricane related facilities and related costs included in the Further Additional Supplemental Appropriations for Disaster Relief and Requirement Act, 2018 (P.L. 115-123).
Appendix A. FDA User Fee Authorizations and Anticipated Collections
Table A-1. FDA User Fee Authorizations and Anticipated Collections
(In Order of FY2018 Anticipated Collections)
|
User Fee |
Initial Authorizing Legislation and Year |
Most Recent Reauthorization and Year, |
FY2018 Anticipated Collections (in Millions of Dollars) |
|
Prescription drug |
Prescription Drug User Fee Act (PDUFA; P.L. 102-300), 1992 |
Food and Drug Administration Reauthorization Act (FDARA; P.L. 115-52), 2017 FY2018-FY2022 |
911 |
|
Tobacco product |
Family Smoking Prevention and Tobacco Control Act (TCA; P.L. 111-31), 2009 |
Indefinite |
672 |
|
Generic drug |
Food and Drug Administration Safety and Innovation Act (FDASIA; P.L. 112-144), 2012 |
Food and Drug Administration Reauthorization Act (FDARA; P.L. 115-52), 2017 FY2018-FY2022 |
494 |
|
Medical device |
Medical Device User Fee and Modernization Act (MDUFMA; P.L. 107-250), 2002 |
Food and Drug Administration Reauthorization Act (FDARA; P.L. 115-52), 2017 FY2018-FY2022 |
193 |
|
Biosimilar |
Food and Drug Administration Safety and Innovation Act (FDASIA; P.L. 112-144), 2012 |
Food and Drug Administration Reauthorization Act (FDARA; P.L. 115-52), 2017 FY2018-2022 |
40 |
|
Mammography |
Mammography Quality Standards Act (MQSA; P.L. P.L. 102-539), 1992 |
Indefinite |
21 |
|
Animal drug |
Animal Drug User Fee Act (ADUFA; P.L. 108-130), 2003 |
Animal Drug and Animal Generic Drug User Fee Amendments of 2018 (P.L. 115-234), 2018 FY2019-2023 |
18 |
|
Color certification |
Color Additive Amendments (P.L. 86-618), 1960 |
Indefinite |
10 |
|
Animal generic drug |
Animal Generic Drug User Fee Act (AGDUFA; P.L. 110-316), 2008 |
Animal Drug and Animal Generic Drug User Fee Amendments of 2018 (P.L. 115-234), 2018 FY2019-2023 |
9 |
|
Rare pediatric disease priority review voucher |
Food and Drug Administration Safety and Innovation Act (FDASIA; P.L. 112-144), 2012 |
Indefinite |
8 |
|
Food reinspection |
Food Safety Modernization Act (FSMA; P.L. 111-353), 2011 |
Indefinite |
6 |
|
Voluntary qualified importer program (VQIP) |
Food Safety Modernization Act (FSMA; P.L. 111-353), 2011 |
Indefinite |
5 |
|
Export certification |
FDA Export Reform and Enhancement Act (P.L. 104-134), 1996 [for medical products]; Food Safety Modernization Act (FSMA; P.L. 111-353), 2011 [for foods] |
Indefinite |
5 |
|
Food and feed recall |
Food Safety Modernization Act (FSMA; P.L. 111-353), 2011 |
Indefinite |
1 |
|
Third party auditor program |
Food Safety Modernization Act (FSMA; P.L. 111-353), 2011 |
Indefinite |
1 |
|
Outsourcing facility |
Drug Quality and Security Act (DQSA; P.L. 113-54), 2013a |
Indefinite |
1 |
|
Tropical disease priority review voucher |
Food and Drug Administration Amendments Act (FDAAA; P.L. 110-85), 2007 |
Indefinite |
— |
|
Medical counter-measures priority review voucher |
21st Century Cures Act (P.L. 114-255), 2016 |
Sunsets October 1, 2023 |
— |
|
Total |
2,397 |
Source: The FY2018 amounts are from the Consolidated Appropriations Act, 2018 (P.L. 115-141) and the funding tables in the Explanatory Statement (Congressional Record, March 22, 2018, vol. 164, no. 50—Book II, pp. H2077-H2078.)
Notes: Individual amounts may not add to the total due to rounding. The user fee amounts in the column "FY2018 Anticipated Collections" are different from the user fee amounts displayed in Table 1. This table presents the total amount authorized for FY2018 from each user fee program, whereas Table 1 displays how the user fees are apportioned across FDA program areas. For example, PDUFA fees contribute to the Human Drugs and Biologics programs, FDA Headquarters, Other Rent and Rent-related activities, and GSA Rental Payments.
a. The Drug Quality and Security Act (P.L. 113-54) authorized FDA to collect fees for the licensure and inspection of certain third-party logistics providers and wholesale drug distributors. According to the FDA FY2019 CJ, this program is still under development.
|
User Fee Authority |
Program |
|||||||||
|
Foods |
Human drugs |
Biologics |
Animal drugs & fees |
Devices & radiological health |
Tobacco |
Headquarters & Commissioner's Office |
GSA rent |
Other rent and rent related |
Not shown by program |
|
|
Prescription drug (PDUFA) |
X |
X |
X |
X |
X |
X |
||||
|
Medical device (MDUFMA) |
X |
X |
X |
X |
X |
|||||
|
Animal drug (ADUFA) |
X |
X |
X |
X |
||||||
|
Animal generic drug (AGDUFA) |
X |
X |
X |
X |
||||||
|
Tobacco (TCA) |
X |
X |
X |
X |
||||||
|
Generic drug (GDUFA) |
X |
X |
X |
X |
X |
|||||
|
Biosimilars (BsUFA) |
X |
X |
X |
X |
X |
|||||
|
MQSA |
X |
X |
||||||||
|
Food reinspection |
X |
X |
X |
X |
||||||
|
Food & feed recall |
X |
X |
X |
X |
||||||
|
VQIP |
X |
X |
X |
X |
||||||
|
Third-party auditor |
X |
X |
X |
X |
X |
|||||
|
Outsourcing facility |
X |
X |
X |
X |
||||||
|
Color certification |
X |
|||||||||
|
Export certification |
X |
|||||||||
|
Priority review vouchers |
X |
|||||||||
|
Med. countermeasures |
X |
|||||||||
Source: Compiled by CRS, using the FY2019 FDA Justification of Estimates for Appropriations Committees.
Notes: The contributions of the user fee authorities to different FDA programs are denoted by "Xs" in the columns.