For centuries, industrial hemp (plant species Cannabis sativa) has been a source of fiber and oilseed used worldwide to produce a variety of industrial and consumer products. Currently, more than 30 nations grow industrial hemp as an agricultural commodity, which is sold on the world market. In the United States, however, production is strictly controlled under existing drug enforcement laws. Currently there is no large-scale commercial production in the United States, and the U.S. market depends on imports.
Congress made significant changes to federal policies regarding hemp in the 2014 farm bill (Agricultural Act of 2014, P.L. 113-79). The 2014 farm bill provided that certain research institutions and state departments of agriculture may grow hemp under an agricultural pilot program. In addition, in subsequent omnibus appropriations, Congress has blocked the U.S. Drug Enforcement Administration (DEA) and federal law enforcement authorities from interfering with state agencies, hemp growers, and agricultural research. Appropriators have also blocked the U.S. Department of Agriculture (USDA) from prohibiting the transportation, processing, sale, or use of industrial hemp that is grown or cultivated in accordance with the 2014 farm bill provision.
Despite these efforts, industrial hemp continues to be subject to U.S. drug laws, and growing industrial hemp is restricted. Under current U.S. drug policy, all cannabis varieties—including industrial hemp—are considered Schedule I controlled substances under the Controlled Substances Act (CSA),1 and DEA continues to control and regulate cannabis production. Although hemp production is now allowed in accordance with the requirements under the 2014 farm bill provision, other aspects of hemp production are still subject to DEA oversight, including the importation of viable seeds.
Congress has sought to further distinguish between industrial hemp and marijuana. Among the bills addressing industrial hemp, the Industrial Hemp Farming Act would amend the CSA to specify that the term marijuana (or marihuana, as it is spelled in the older statutes) does not include industrial hemp, thus excluding hemp from the CSA as a controlled substance subject to DEA regulation. This bill was reintroduced and expanded from bills introduced in previous Congresses dating back to the 109th Congress. An expanded version of this bill was introduced in the 115th Congress in both the House and Senate (H.R. 5485; S. 2667). Other provisions in these bills would further facilitate hemp production in the United States. Many of the provisions in these bills are included in the Senate version of the 2018 farm bill legislation (S. 3042) that is now being debated in Congress. Similar provisions are not part of the House version of the 2018 farm bill (H.R. 2).
Other introduced legislation would amend the CSA "to exclude cannabidiol and cannabidiol-rich plants from the definition of marihuana" intended to promote the possible medical applications of industrial hemp. Myriad other bills introduced in both the House and the Senate would further amend the CSA and other federal laws to address industrial hemp.
Hemp Production and Use
Botanically, industrial hemp and marijuana are from the same species of plant, Cannabis sativa, but from different varieties or cultivars that have been bred for different uses.2 However, industrial hemp and marijuana are genetically distinct forms of cannabis3 that are distinguished by their use, chemical makeup, and differing cultivation practices in production. While marijuana generally refers to the psychotropic drug (whether used for medicinal or recreational purposes), industrial hemp is cultivated for use in the production of a wide range of products, including foods and beverages, personal care products, nutritional supplements, fabrics and textiles, paper, construction materials, and other manufactured goods.
Both hemp and marijuana also have separate definitions in statute. While marijuana is defined in U.S. drug laws, Congress established a statutory definition for industrial hemp as "the plant Cannabis sativa L. and any part of such plant, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis" as part of the 2014 farm bill.4 Hemp is generally characterized by plants that are low in delta-9 tetrahydrocannabinol (delta-9 THC), the dominant psychotrophic ingredient in Cannabis sativa.5
For more background information, see CRS Report R44742, Defining "Industrial Hemp": A Fact Sheet. However, joint guidance issued in August 2016 by DEA, USDA, and the Food and Drug Administration (FDA) suggests that there continues to be questions about what constitutes industrial hemp and its oversight under federal law.
Commercial Uses of Hemp
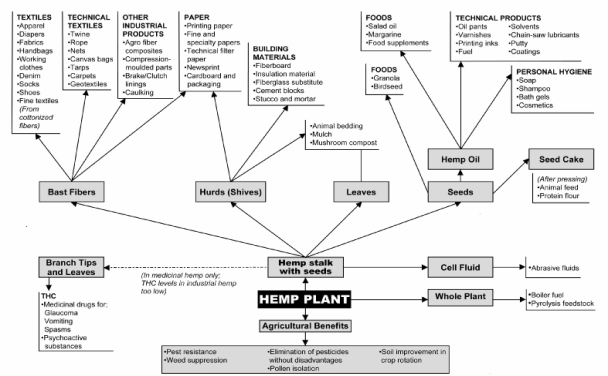
The global market for hemp consists of more than 25,000 products in nine submarkets: agriculture, textiles, recycling, automotive, furniture, food and beverages, paper, construction materials, and personal care (Table 1). Hemp can be grown as a fiber, seed, or dual-purpose crop.6 The stalk and seed are the harvested products. The interior of the stalk has short woody fibers called hurds; the outer portion has long bast fibers. Hemp seed/grains are smooth and about one-eighth to one-fourth of an inch long.7
Hemp fibers are used in fabrics and textiles, yarns and spun fibers, paper, carpeting, home furnishings, construction and insulation materials, auto parts, and composites. Hurds are used in animal bedding, material inputs, papermaking, and oil absorbents. Hemp seed and oilcake are used in a range of foods and beverages (e.g., salad and cooking oil and hemp dairy alternatives) and can be an alternative food and feed protein source.8 Oil from the crushed hemp seed is used in soap, shampoo, lotions, bath gels, and cosmetics.9 Hemp is also being used in nutritional supplements and in medicinal and therapeutic products, including pharmaceuticals. It is also used in a range of composite products. Hempcrete (a mixture of hemp hurds and lime products) is being used as a building material. Hemp is also used as a lightweight insulating material and in hemp plastics and related composites for use as a fiberglass alternative by the automotive and aviation sectors.10 Hemp is also promoted as a potential biodiesel feedstock11 and cover crop.
These types of commercial uses are widely documented in a range of feasibility and marketing studies conducted by researchers at USDA and various land grant universities and state agencies. (A listing of these studies is in the Appendix A.) Currently, finished hemp products and raw material inputs are mostly imported into the United States and sold for use in further processing and manufacturing for a wide range of products.
 |
|
Source: Industrial Hemp Association of Tasmania, http://www.ihat.org.au/. Notes: Other hemp product charts include D. G. Kraenzel et al., "Industrial Hemp as an Alternative Crop in North Dakota," AER-402, North Dakota State University, July 23, 1998; and National Hemp Association, http://nationalhempassociation.org/. |
Estimated Retail Market
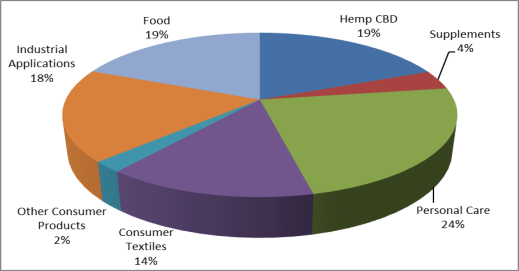
No official estimates are available of the value of U.S. sales of hemp-based products. The Hemp Industries Association (HIA) reports total U.S. retail sales of hemp products of nearly $700 million in 2016,12 which includes food and body products, dietary supplements, clothing, auto parts, building materials, and other consumer products (Figure 2). HIA claims that U.S. hemp retail sales have increased by about 10% to more than 20% annually since 2011. Much of this growth is attributable to sales of hemp-based body products, supplements, and foods. Combined, these categories accounted for more than two-thirds of the value of U.S. retail sales in 2016.
Little detailed information is available on some other hemp-based sectors, such as for use in construction, biofuels, paper, textiles, or other manufacturing uses. Data are also not available on existing businesses or processing facilities.
|
Source: HIA, "2015 Annual Retail Sales for Hemp Products Estimated at $573 Million," May 9, 2016. |
 |
|
Source: HIA, "2015 Annual Retail Sales for Hemp Products Estimated at $573 Million," May 9, 2016. |
U.S. Hemp Imports
Hemp imports to the United States—consisting of hemp seeds and fibers often used as inputs for use in further manufacturing—totaled $67.3 million in 2017 (Table 1). Although hemp imports have declined from a record high of $78.1 million in 2015, U.S. hemp imports have steadily increased since 2005 when hemp imports totaled $5.7 million. This increase in trade followed the resolution of a legal dispute over U.S. imports of hemp foods in late 2004 (see "Dispute over Hemp Imports (1999-2004)") and also prior prohibitions on U.S. domestic production.
In 2017, nearly two-thirds (64%) of the value of all U.S. hemp imports were of hemp seeds, which were used mostly as inputs and ingredients for hemp-based products. Other ingredient imports—hemp oil, seed cake, and solids—accounted for another 28% of the value of total imports. Import hemp yarns and fibers accounted for about 8% of total import value in 2017 (Table 1). Trade data are not available for finished products, such as hemp-based clothing or other products including construction materials, carpets, or paper products.
|
|
Units |
1996 |
2000 |
2005 |
2010 |
2013 |
2014 |
2015 |
2016 |
2017 |
|
Hemp Seeds |
$1000 |
— |
— |
271 |
5,125 |
26,942 |
29,326 |
54,191 |
51,018 |
42,897 |
|
Hemp Oil and Fractions |
$1000 |
— |
2,822 |
3,027 |
1,833 |
2,264 |
3,446 |
4,836 |
6,142 |
7,603 |
|
Hemp Seed Oilcake and Solids (HS 2306900130) |
$1000 |
— |
— |
— |
2,369 |
6,279 |
8,159 |
16,281 |
8,620 |
11,494 |
|
True Hemp, raw/proc. not spun (HS 5302) |
$1000 |
100 |
577 |
228 |
94 |
78 |
114 |
292 |
690 |
780 |
|
True Hemp Yarn (HS 5308200000) |
$1000 |
25 |
640 |
904 |
296 |
482 |
909 |
1,497 |
1,867 |
2,739 |
|
True Hemp Woven Fabrics (HS 5311004010) |
$1000 |
1,291 |
2,258 |
1,232 |
1,180 |
1,057 |
900 |
1,020 |
744 |
1,819 |
|
Total |
1,416 |
6,297 |
5,662 |
10,897 |
37,102 |
42,854 |
78,117 |
69,081 |
67,332 |
|
|
Hemp Seeds (HS 1207990320) |
metric ton |
— |
— |
92 |
712 |
2,311 |
2,783 |
15,977 |
17,820 |
7,606 |
|
Hemp Oil and Fractions (HS 1515908010) |
metric ton |
— |
587 |
287 |
215 |
450 |
1,155 |
538 |
767 |
749 |
|
Hemp Seed Oilcake and Solids (HS 2306900130) |
metric ton |
— |
— |
— |
240 |
601 |
938 |
1,826 |
1,163 |
1,475 |
|
True Hemp, raw/proc. not spun (HS 5302) |
metric ton |
53 |
678 |
181 |
42 |
72 |
161 |
278 |
494 |
621 |
|
True Hemp Yarn (HS 5308200000) |
metric ton |
6 |
89 |
113 |
42 |
70 |
102 |
166 |
213 |
312 |
|
Subtotal |
59 |
1,354 |
673 |
1,251 |
3,504 |
5,139 |
18,785 |
20,457 |
10,763 |
|
|
True Hemp Woven Fabrics (HS 5311004010) |
m2 (1000) |
435 |
920 |
478 |
284 |
224 |
151 |
206 |
150 |
360 |
Source: Compiled by CRS using data from the U.S. International Trade Commission, http://dataweb.usitc.gov. Data are by Harmonized System (HS) code. Data shown as "—" indicate data are not available as breakout categories or, for some product subcategories, were established only recently. Data are not adjusted for inflation.
Notes: Historical data for hemp seeds combine reported statistics for three HTS categories: HTS 1207990320 (2012-present), HTS 1207990020 (2007-2011) and HTS 1207990120 (2005-2006). Data for hemp oil combine HTS 15150904010 (1999-2001) and HTS 15159008010 (2002-present).
Canada is the single largest supplier of U.S. hemp imports, accounting for about 90% of the value of annual imports. Other leading country suppliers include China (about 3-5% of annual imports) and Romania (2-4%). Remaining imports are supplied by other European countries, India, the Dominican Republic, and Chile. Canada is the primary source of U.S. imports of food-grade hemp seed and oilcake, with supplies also from China and Europe. China and some European countries are major suppliers of raw and processed hemp fiber and yarn.
Three forms of seed are imported:13 (1) de-hulled seed, often referred to as hemp hearts, hulled seeds, or hemp nut, used in a range of food products; (2) non-viable whole seed, rendered non-viable through a sterilization process, usually through temperature exposure; and (3) viable whole seed, capable of germination under suitable conditions. Most hemp seed cultivars originate in Europe (France, Germany, Hungary, Italy, Poland, and Romania), Russia, Ukraine, and China.
U.S. Market Potential
Most researchers acknowledge the potential profitability of industrial hemp, but also the potential obstacles to its development. Current challenges facing the industry include the need to re-establish agricultural supply chains, breed varieties with modern attributes, upgrade harvesting equipment, modernize processing and manufacturing, and identify new market opportunities.14
In the past two decades, researchers at the USDA and various land grant universities and state agencies (for example, Arkansas, Kentucky, Maine, Minnesota, North Dakota, Oregon, and Vermont; see Appendix A) have conducted several feasibility and marketing studies. More recent available market reports indicate that the estimated gross value of hemp production per acre is about $21,000 from seeds and $12,500 from stalks.15
Studies by researchers in Canada and various state agencies provide a mostly positive market outlook for growing hemp, citing rising consumer demand and the potential range of product uses for hemp. Some state reports claim that if current restrictions on growing hemp in the United States were removed, agricultural producers in their states could benefit. A 2008 study reported that acreage under cultivation in Canada, "while still showing significant annual fluctuations, is now regarded as being on a strong upward trend." Most studies generally note that hemp "has such a diversity of possible uses, [and] is being promoted by extremely enthusiastic market developers." Other studies highlight certain production advantages associated with hemp or acknowledge hemp's benefits as a rotational crop or further claim that hemp may be less environmentally degrading than other agricultural crops. Other studies claim certain production advantages to hemp growers, such as relatively low input and management requirements.
Other studies differ from the various state reports and provide a less favorable aggregate view of the potential market for hemp growers in the United States, highlighting challenges facing U.S. growers. For example, a 2000 study by USDA projected that U.S. hemp markets "are, and will likely remain, small, thin markets." It also cited "uncertainty about long-run demand for hemp products and the potential for oversupply" among possible downsides of potential future hemp production.16 Similarly, a study by University of Wisconsin-Madison concluded that hemp production "is not likely to generate sizeable profits," and, although hemp may be "slightly more profitable than traditional row crops," it is likely "less profitable than other specialty crops" due to the "current state of harvesting and processing technologies, which are quite labor intensive, and result in relatively high per unit costs."17 The study also noted that U.S. growers could be affected by competition from other world producers and by production limitations in the United States, including yield variability and lack of harvesting innovations and processing facilities, as well as difficulty transporting bulk hemp. The study further claimed that most estimates of profitability from hemp production are highly speculative and often do not include additional costs of growing hemp in a regulated market, such as the cost associated with "licensing, monitoring, and verification of commercial hemp."
A 2013 study by researchers at the University of Kentucky predicted that despite "showing some positive returns, under current market conditions, it remained unclear whether anticipated hemp returns would be large enough to entice Kentucky grain growers to shift out of grain production" under most circumstances. They also noted that "short run employment opportunities evolving from a new Kentucky hemp industry appear limited (perhaps dozens of new jobs, not 100s)," because of continued uncertainty in the industry.18 Overall, the study concluded that there were many remaining unknowns and that further analysis and production research was needed.
A 2016 study notes that the most promising markets for North American hemp production is a continued focus on oilseed production and cannabidiol (CBD), a non-intoxicant cannabinoid that has promise for its therapeutic use as a pharmaceutical product.19
Given the absence since the 1950s of any commercial and unrestricted hemp production in the United States, it is not possible to predict with any degree of confidence the potential market and employment effects of relaxing current restrictions on U.S. hemp production. While expanded market opportunities might exist in some states or localities if current restrictions on production are lifted, it is not possible to predict the potential for future retail sales or employment gains in the United States, either nationally or within certain states or regions. Information on these types of probable effects is not available from previous market analyses that have been conducted by researchers at USDA and land grant universities and state agencies.
Global Production
International Production
Approximately 30 countries in Europe, Asia, and North and South America currently permit farmers to grow hemp. Aggregated production data from the United Nations do not include all countries (most notably Canada) and may differ from other sources but comprise the most readily available source of information. Based on these data, excluding Canada, global acreage in hemp cultivation in 2016—both hemp seed and hemp tow waste—is reported at about 192,000 acres (Figure 3), with a reported total production of 355 million pounds (Figure 4). United Nations data do not include Canada, which is a major hemp producing and exporting country. Including other data for Canada, in 2016, aggregate acreage totaled at about 225,000 acres. Canada is also major supplier of U.S. hemp imports, particularly of hemp-based foods and food ingredients and other related imported products.
Preliminary information for 2017 indicate that hemp acreage in Canada and the European Union (EU) countries reached record levels, which could put global acreage at more than 330,000 acres. Still, as a share of total crop production in these countries, hemp production accounts for a negligible share (less than 0.5%) of total acreage.
|
|
|
Global Production (Excluding Canada)
Leading global hemp producers include Europe, China, South Korea, and Russia. Some countries never outlawed production; other countries banned production for certain periods in the past and later lifted these restrictions. Hemp production across these countries and regions account for nearly all the reported production and acreage reported in the U.N. database.
According to Food and Agriculture Organization (FAO) of the United Nations data, Europe is the world's single largest hemp producing market. In 2016, European countries produced hemp on more than 80,000 acres—a record high20 and accounting for about 40% of FAO-reported global acreage. The EU has an active hemp market, with production in most member nations. Production is centered in France, the Netherlands, Lithuania, and Romania.21 Many EU countries lifted their bans on hemp production in the 1990s and, until recently, also subsidized the production of "flax and hemp" under the EU's Common Agricultural Policy.22 Most EU production is of hurds, seeds, fibers, and pharmaceuticals.23 Other non-EU European countries with reported hemp production include Russia, Ukraine, and Switzerland.
China is another major producer, mostly of hemp textiles and related products, as well as a major supplier to the United States. In 2016, China's hemp was grown on about 20,000 acres. FAO data also report hemp production in Chile, China, Iran, Japan, South and North Korea, Pakistan, Russia, Syria, and Turkey. Other countries with active hemp grower and/or consumer markets not included in FAO's annual compilation are New Zealand, India, Egypt, South Africa, Thailand, Malawi, and Uruguay.
Production in Canada
Canada's commercial hemp industry is fairly new: Canada began to issue licenses for research crops in 1994, followed by commercial licenses starting in 1998. Since hemp cultivation was legalized in Canada, production has been variable year to year (Figure 5) but generally increasing—which some attribute to increased import demand in the United States.24 Acreage has ranged from 48,000 planted acres in 2006 to about 8,000 acres in 2008, rising again to a 100,000 acres in 2014 but then sharply dropping back again to 33,000 acres in 2016. In 2017, acreage in hemp cultivation and production rose sharply—reaching a record of nearly 140,000. Canada's hemp cultivation still accounts for only about 1% of the country's available farmland. The number of cultivation licenses has also varied from year to year, reaching a high of 560 licenses in 2006, followed by a low of 77 licenses in 2008 and rising to 340 licenses in 2011.25 Since then, the number of licenses has risen to more than 1,100 issued in 2015 and 2016. Annual retail sales of all Canadian-derived hemp seed products are estimated between $20 million and $40 million, and the number of businesses active in the sector has grown over the past few years.26
|
Source: CRS from Agriculture and Agri-Food Canada data, "Industrial Hemp Statistics," and "Industrial Hemp Production in Canada," and other press reports (D. Brown, "Canada on Course for Record Hempseed Crop in 2017," June 2017). Note: The downturn in 2007 is viewed as a correction of overproduction in 2006 following the "success of the court case against DEA in 2004, and continued improvements in breeding, production, and processing," which resulted in part in a "dramatic reduction in hemp acreage planted" in 2007. The 2007 downturn is also attributed to "increasingly positive economics of growing other crops" (Manitoba Agriculture, National Industrial Hemp Strategy, March 2008, prepared for Food and Rural Initiative Agriculture and Agri-Food Canada). |
The development of Canada's hemp market followed a 60-year prohibition and is strictly regulated.27 The Office of Controlled Substances of Health Canada, which issues licenses for all activities involving hemp administers the program. Under the regulation, all industrial hemp grown, processed, and sold in Canada may contain THC levels of no more than 0.3% of the weight of leaves and flowering parts. Canada has also set a maximum level of 10 parts per million for THC residues in products derived from hemp grain, such as flour and oil.28 To obtain a license to grow hemp, Canadian farmers must submit extensive documentation, including background criminal record checks, the Global Positioning System (GPS) coordinates of their fields, and supporting documents (from the Canadian Seed Growers' Association or the Canadian Food Inspection Agency) regarding their use of certified low-THC hemp seeds and approved cultivars; and they must allow government testing of their crop for THC levels.29
In 2016, Canada further relaxed its regulations of industrial hemp production by amending its drug laws to provide for a "class exemption" for hemp in order to "simplify the license application process for the 2017 growing season."30 According to Health Canada, the Section 56 Class Exemption "better aligns regulation of industrial hemp with the demonstrated low public health and safety risks of the crop" intended "to simplify the license application process" as Canada moves forward with "its commitment to legalize, strictly regulate, and restrict access to marijuana."31 Among the types of simplifications and streamlining are:
- Reduced pre-requisite requirements (e.g., no longer need to pre-identify planting sites, no more minimum acreage requirements);
- Reduced paperwork (to a single form), reduced proof requirements (to a single attestation), and growers may now apply electronically;
- THC testing requirements mostly eliminated (except for pedigreed seed or applications to be added to the list of approved cultivars);
- License expiry date extended until March the following year; and
- Criminal record check valid now for one year.
The potential impact could greatly facilitate hemp production for Canadian farmers, which could continue to give them an advantage over U.S. growers, where hemp production remains restricted and legal in only few cases.
U.S. Production
Following enactment of the 2014 farm bill, hemp cultivation became allowed under certain circumstances by research institutions and state departments of agriculture. Official estimates of U.S. hemp production are not available. Information compiled by states and industry indicate that there were more than 25,500 acres of hemp production in 2017, up from 9,770 acres in 2016 (Table 2). In 2017, there were 1,420 registered or licensed growers and 32 universities conducting hemp research nationwide.32 Investment in hemp processing facilities is underway in several states, including Kentucky,33 Tennessee,34 North Carolina,35 and New York.36
|
State |
Number Production Acres |
Purposes Grown |
|
|
2016 |
2017 |
||
|
Colorado |
5,921 |
9,700 |
Fiber, grain, seed for sale, CBD |
|
Hawaii |
1 |
TBD |
NA |
|
Indiana |
2 |
5 |
NA |
|
Kentucky |
2,525 |
3,100 |
Fiber, grain, seed for sale, CBD |
|
Maine |
1 |
30 |
Unknown |
|
Minnesota |
51 |
1,205 |
Fiber, grain, CBD (non-medical) |
|
Montana |
0 |
542 |
|
|
Nebraska |
1 |
1 |
NA |
|
Nevada |
216 |
417 |
Fiber, grain, CBD |
|
New York |
30 |
2,000 |
NA |
|
North Carolina |
0 |
965 |
|
|
North Dakota |
70 |
3,020 |
Grain |
|
Oregon |
500 |
3,469 |
NA |
|
Pennsylvania |
0 |
36 |
NA |
|
Tennessee |
225 |
200 |
CBD |
|
Vermont |
180 |
575 |
CBD research |
|
Virginia |
37 |
87 |
Fiber, grain research |
|
Washington |
0 |
175 |
NA |
|
West Virginia |
10 |
14 |
Fiber, grain |
|
Total |
9,770 |
25,541 |
|
Source: CRS from information from Vote Hemp, "2017 U.S. Hemp Crop Report," January 2018 (number of acres), and the Colorado Department of Agriculture, "2016 National Hemp Regulatory Meeting Survey," October 2016 ("purposes grown"). "NA" indicates that information is not available.
Hemp was widely grown in the United States from the colonial period into the mid-1800s. Fine and coarse fabrics, twine, and paper from hemp were in common use. By the 1890s, labor-saving machinery for harvesting cotton made the latter more competitive as a source of fabric for clothing, and the demand for coarse natural fibers was met increasingly by imports. Industrial hemp was handled in the same way as any other farm commodity in that USDA compiled statistics and published crop reports37 and provided assistance to farmers promoting production and distribution.38 In the early 1900s, hemp continued to be grown, and USDA researchers continued to publish information related to hemp production and also reported on hemp's potential for use in textiles and in paper manufacturing.39 Several hemp advocacy groups, including HIA and Vote Hemp, Inc., have compiled other historical information and have copies of original source documents.40
Between 1914 and 1933, in an effort to stem the use of Cannabis flowers and leaves for their psychotropic effects, 33 states passed laws restricting legal production to medicinal and industrial purposes only.41 The 1937 Marihuana Tax Act defined hemp as a narcotic drug, requiring that farmers growing hemp hold a federal registration and special tax stamp, effectively limiting further production expansion.
In 1943, U.S. hemp production reached more than 150 million pounds (140.7 million pounds hemp fiber; 10.7 million pound hemp seed) on 146,200 harvested acres. This compared to pre-war production levels of about 1 million pounds. After reaching a peak in 1943, production started to decline. By 1948, production had dropped back to 3 million pounds on 2,800 harvested acres, with no recorded production after the late 1950s.42
Federal Law and Requirements
Controlled Substances Act of 1970
In 1937, Congress passed the first federal law to discourage cannabis production for marijuana while still permitting industrial uses of the crop (the Marihuana Tax Act; 50 Stat. 551). Under this statute, the government actively encouraged farmers to grow hemp for fiber and oil during World War II. After the war, competition from synthetic fibers, the Marihuana Tax Act, and increasing public anti-drug sentiment resulted in fewer and fewer acres of hemp being planted and none at all after 1958. The Controlled Substances Act of 1970 (CSA, 21 U.S.C. §801 et. seq.) placed the control of select plants, drugs, and chemical substances under federal jurisdiction and was enacted, in part, to replace previous federal drug laws with a single comprehensive statute.43
The CSA adopted the same definition of Cannabis sativa that appeared in the 1937 Marihuana Tax Act. The definition of "marihuana" (21 U.S.C. §802(16)) reads:
The term marihuana means all parts of the plant Cannabis sativa L., whether growing or not; the seeds thereof; the resin extracted from any part of such plant; and every compound, manufacture, salt, derivative, mixture, or preparation of such plant, its seeds or resin. Such term does not include the mature stalks of such plant, fiber produced from such stalks, oil or cake made from the seeds of such plant, any other compound ... or preparation of such mature stalks (except the resin extracted therefrom), fiber, oil, or cake, or the sterilized seed of such plant which is incapable of germination.
The statute thus retains control over all varieties of the cannabis plant by virtue of including them under the term marihuana and does not distinguish between low- and high-THC varieties. The language exempts from control the parts of mature plants—stalks, fiber, oil, cake, etc.—intended for industrial uses. Some have argued that the CSA definition exempts industrial hemp under its term exclusions for stalks, fiber, oil, cake, and seeds.44 DEA refutes this interpretation.45
Strictly speaking, CSA does not make growing cannabis illegal; rather, it places strict controls on its production, making it illegal to grow the crop without a DEA permit. Regarding industrial hemp, however, growers that comply with the 2014 farm bill provision (discussed in the next section) do not need DEA approval.
Agricultural Act of 2014
The 113th Congress considered various changes to U.S. policies regarding industrial hemp during the omnibus farm bill debate.46 The 2014 farm bill (Agricultural Act of 2014 [P.L. 113-79], §7606)47 provides that certain "institutions of higher education"48 and state departments of agriculture may grow industrial hemp, as part of an agricultural pilot program, if allowed under state laws where the institution or state department of agriculture is located. The farm bill also established a statutory definition of industrial hemp as "the plant Cannabis sativa L. and any part of such plant, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis." The provision was included as part of the research title of the law. The provision did not include an effective date that would suggest any kind of program rollout, and there appears to be nothing in the conference report or bill language to suggest that the states might not be able to immediately initiate action on this provision.
This provision was adopted when Representatives Polis, Massie, and Blumenauer introduced an amendment to the House version of the farm bill (H.R. 1947, the Federal Agriculture Reform and Risk Management Act of 2013) during floor debate on the bill. The amendment (H.Amdt. 208) was to allow institutions of higher education to grow or cultivate industrial hemp for the purpose of agricultural or academic research and applied to states that already permit industrial hemp growth and cultivation under state law. The amendment was adopted by the House of Representatives. Although the full House ultimately voted to reject H.R. 1947, similar language was included as part of a subsequent revised version of the House bill (H.R. 2642), which was passed by the full House.
In the Senate, Senators Wyden, McConnell, Paul, and Merkley introduced an amendment to the Senate version of the farm bill (S. 954, the Agriculture Reform, Food and Jobs Act of 2013). The amendment (S.Amdt. 952) would have amended the CSA to exclude industrial hemp from the definition of marijuana. The amendment was not adopted as part of the Senate-passed farm bill.
During conference on the House and Senate bills, the House provision was adopted with additional changes. The enacted law expands the House bill provision to allow both certain research institutions and also state departments of agriculture to grow industrial hemp, as part of an agricultural pilot program, if allowed under state laws where the institution or state department of agriculture is located.
As the farm bill did not include an effective date distinct from the date of enactment, several states responded by making immediate plans to initiate new hemp pilot projects. In addition, several states enacted legislation to allow for hemp cultivation, which is a precondition for allowances under the 2014 farm bill.
Some have speculated whether the industrial hemp provision in the 2014 farm bill could terminate, expire, or require reauthorization in a subsequent farm bill.49 Although some individual authorizations in the farm bill specifically have provisions indicating that they expire in 2018 (such as authorized funding levels), the industrial hemp research provision in the 2014 farm bill does not have such language. Furthermore, the farm bill does not contain a default sunset provision for all its authorizations. Accordingly, the industrial hemp research provision in the 2014 farm bill appears to be intended to have some degree of permanence.
Despite these efforts, industrial hemp continues to be subject to U.S. drug laws, and growing industrial hemp is restricted. Under current U.S. drug policy, all cannabis varieties—including industrial hemp—are considered Schedule I controlled substances under the Controlled Substances Act (CSA, 21 U.S.C. §§801 et seq.). Although hemp production is now allowed in accordance with the requirements under the 2014 farm bill provision, other aspects of production are still subject to DEA oversight, including the importation of viable seeds, which requires DEA registration according to the Controlled Substances Import and Export Act (CSIEA, 21 U.S.C. §§951-971). This requirement was reinforced in a 2016 joint "Statement of Principles on Industrial Hemp" from DEA, USDA, and FDA.50 The 2016 guidance also clarifies DEA's contention that the commercial sale or interstate transfer of hemp continues to be restricted. (For more information, see "2016 Joint "Statement of Principles" on Industrial Hemp".)
Selected Appropriations Actions
Immediately following the 2014 farm bill, some states quickly responded by expanding their efforts to grow industrial hemp. However, these initiatives were slowed by the absence of viable seeds in the United States to grow industrial hemp and DEA actions blocking the importation of viable seed. (For more information, see "DEA's Blocking of Imported Viable Hemp Seeds".) To avoid future similar DEA actions that might further stall full implementation of the hemp provision of the farm bill, Congress acted swiftly. Both the House and Senate FY2015 Commerce-Justice-Science (CJS) appropriations bills contained provisions to block federal law enforcement authorities from interfering with state agencies and hemp growers and counter efforts to obstruct agricultural research. The enacted FY2015 appropriation blocked federal law enforcement authorities from interfering with state agencies, hemp growers, and agricultural research.51 The provision stated that "none of the funds made available" to the U.S. Justice Department and DEA "may be used in contravention" of the 2014 farm bill. Similar language has been included in each subsequent enacted CSJ appropriations and is now also part annual Agriculture appropriations.
The enacted FY2018 Agriculture appropriation states that none of the funds made available by the Agriculture or any other appropriation may be used in contravention of the 2014 farm bill provision or "to prohibit the transportation, processing, sale, or use of industrial hemp that is grown or cultivated" in accordance with the farm bill provision "within or outside the State in which the industrial hemp is grown or cultivated."52 The FY2017 and FY2016 Agriculture appropriation contained similar language.53 Language referring to selling industrial hemp within a state addresses intrastate commerce, whereas language referring to selling hemp outside the state may be considered to address interstate commerce.
The FY2018 CJS appropriation (Division B of P.L. 115-31) states that "none of the funds made available by this Act may be used in contravention of section 7606 (''Legitimacy of Industrial Hemp Research'') of the Agricultural Act of 2014 (P.L. 113-79) by the Department of Justice or the Drug Enforcement Administration." The enacted FY2017, FY2016, and FY2015 CJS appropriation contained similar language to block federal law enforcement from interfering with state agencies, hemp growers, and agricultural research.54
Other proposed appropriations bills had also addressed industrial hemp. For example, the Senate FY2018 Energy and Water Development and Related Agencies appropriation proposed to prohibit regulators from denying hemp growers access to water if hemp is grown or cultivated in accordance with the laws of the state in which such use occurs.55 The provision was not enacted as part of the omnibus appropriation.
In prior appropriations debates, the House CJS bills also included provisions stating that no funds be used to prevent a state from implementing its own state laws that "authorize the use, distribution, possession, or cultivation of industrial hemp" as defined in the 2014 farm bill.56 These provisions were not adopted. In addition, as part of the FY2017 Agriculture appropriations debate, the Senate committee report urged USDA "to clarify the Agency's authority to award Federal funds to research projects deemed compliant with Section 7606 of the Agricultural Act of 2014."57 The latter provision addressed questions by a number of state and private research institutions about the extent to which industrial hemp initiatives might be eligible for U.S. federal grant programs (both USDA and non-USDA program funds). This action built on previous efforts by several Members of Congress who sent a letter to USDA in November 2015 requesting clarification of the agency's research funds for industrial hemp.58
Additional information on the legislative intent behind the 2014 farm bill provision and a congressional response to DEA has taken actions that are in contravention of the farm bill were articulated in an amicus brief filed by Members of Congress in HIA, et al., v. DEA, et al.59
State Laws
Since the mid-1990s, there has been a resurgence of interest in the United States in producing industrial hemp. Farmers in regions of the country that are highly dependent upon a single crop, such as tobacco or wheat, have shown interest in hemp's potential as a high-value alternative crop, although the economic studies conducted so far paint a mixed profitability picture. Beginning around 1995, an increasing number of state legislatures began to consider a variety of initiatives related to industrial hemp. Most of these have been resolutions calling for scientific, economic, or environmental studies, and some are laws authorizing planting experimental plots under state statutes.
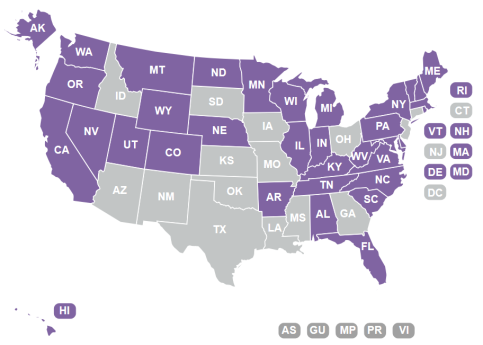
Following enactment of the 2014 farm bill provision, several states quickly adopted new state laws to allow for cultivation. To date, nearly 40 states or territories have enacted or introduced legislation favorable to hemp cultivation (Figure 6). Other states reportedly considering hemp legislation include Arizona, Georgia, Iowa, Kansas, Mississippi, New Mexico, Oklahoma, South Dakota, and Texas.60 (The status of state actions regarding hemp is changing rapidly, and information differs depending on source.61)
Requirements differ among the states, and some states have enacted laws that are considered more comprehensive than others.62 Some common provisions across these state laws include:
- defining industrial hemp (based on the percentage of THC it contains) and excluding hemp from the definition of controlled substances under state law;
- authorizing the growing and possessing of industrial hemp by creating an advisory board or commission;
- establishing or authorizing a state licensing or registration program for growers and/or seed breeders;
- requiring recordkeeping;
- requiring waivers in some cases;
- establishing or authorizing fee structures;
- establishing inspection procedures;
- allowing state departments to collect funds for research programs;
- promoting research and development of markets for industrial hemp;
- establishing certified seed requirements63 or, in some states, "heritage hemp seeds" (e.g., in Colorado and Kentucky); and
- establishing penalties.
Some states have well-developed guidelines for growers, covering issues such as registration and reporting requirements, inspection, THC testing and threshold determination, seed availability and certification, pesticide use, production standards, and other information. Other general requirements may apply under some circumstances. For example, in 2016, USDA published guidance on organic certification of industrial hemp products.64 Some are calling for the need to develop more far-reaching consensus standards for a range of cannabis varieties given concerns about the general lack of standards and test methods.65 Production of industrial hemp has been reported in several states (Table 2).
 |
|
Source: National Conference of State Legislatures, State Industrial Hemp Statutes (http://www.ncsl.org/research/agriculture-and-rural-development/state-industrial-hemp-statutes.aspx). Accessed May 29, 2018. Notes: Darker shade indicates "allows cultivation of hemp for commercial, research or pilot programs." Non-shaded states indicate "does not allow cultivation of hemp." |
Among the states that have enacted taxation and/or fees for industrial hemp are California, Colorado, Indiana, Kentucky, Maine, Montana, Nevada, North Dakota, Oregon, Tennessee, Vermont, and West Virginia.66
DEA Policy Statements and Other Federal Guidance
DEA Permit Requirements
Federal law prohibits cultivation of cannabis without a permit, and DEA enforces standards governing the security conditions under which the crop must be grown. In other words, a grower needs to get permission from DEA to grow cannabis or faces the possibility of federal charges or property confiscation, regardless of whether the grower has a state-issued permit.67
Prior to the 2014 farm bill, although many states had established programs under which a farmer may be able to grow industrial hemp under certain circumstances, a grower would still need to obtain a DEA permit and abide by DEA's strict production controls. This situation resulted in some high-profile cases in which growers applied for a permit but DEA did not approve (or denied) a permit to grow hemp, even in states that authorize cultivation under state laws.
Even if DEA were to approve a permit, production might be discouraged because of the perceived difficulties of working through DEA licensing requirements and installing the types of structures necessary to obtain a permit. Obtaining a DEA permit required that the applicant demonstrate that an effective security protocol will be in place at the production site, such as security fencing around the planting area, a 24-hour monitoring system, controlled access, and possibly armed guards to prevent public access.68 DEA application requirements also include a nonrefundable fee, FBI background checks, and extensive documentation. It could also be argued that the necessary time-consuming steps involved in obtaining and operating under a DEA permit, the additional management and production costs from installing structures, and other business and regulatory requirements could ultimately limit the operation's profitability.
There was also ongoing tension between federal and state authorities over state hemp policies. After North Dakota passed its own state law authorizing industrial hemp production in 1999,69 researchers repeatedly applied for, but did not receive, a DEA permit to cultivate hemp for research purposes in the state.70 Also in 2007, two North Dakota farmers were granted state hemp farming licenses and, in June 2007, filed a lawsuit in U.S. District Court (North Dakota) seeking "a declaratory judgment" that the CSA "does not prohibit their cultivation of industrial hemp pursuant to their state licenses."71 The case was dismissed in November 2007.72 The case was appealed to the U.S. Court of Appeals (8th Circuit) but was again dismissed in December 2009.73
As some states began to allow U.S. producers to grow hemp under state law, some growers were foregoing the requirement to obtain a federal permit. For example, in 2009, Montana's Agriculture Department issued its first state license for an industrial hemp-growing operation in the state, and media reports indicated that the grower did not intend to request a federal permit.74 Such cases posed a challenge to DEA of whether it was willing to override the state's authority to allow for hemp production in the state.
|
Other DEA Policies Regarding Industrial Hemp (Pre-2014 Farm Bill) DEA documentation illustrates how DEA has reviewed inquiries about the legal status of hemp-based products, including inquiries from U.S. customs inspectors regarding the need for guidance regarding imported hemp products: DEA took the position that it would follow the plain language of the Controlled Substances Act (CSA), which expressly states that anything that contains "any quantity" of marijuana or THC is a schedule I controlled substance. However, as a reasonable accommodation, DEA exempted from control legitimate industrial products that contained THC but were not intended for human consumption (such as clothing, paper, and animal feed). DEA's position that "anything that contains 'any quantity' of marijuana or THC" should be regarded as a controlled substance is further supported by reports published by the National Institute on Drug Abuse, which is part of the National Institutes of Health. Although it does not have a formal position about industrial hemp, its research tends to conflate all cannabis varieties, including marijuana and hemp. For example, it reports: "All forms of marijuana are mind-altering (psychoactive)," and "they all contain THC (delta-9-tetrahydrocannabinol), the main active chemical in marijuana." DEA further maintained that the CSA does not differentiate between different varieties of cannabis based on THC content. Regarding interest among growers in some states to cultivate hemp for industrial use, DEA claimed that the courts have supported the agency's current policy that all hemp growers—regardless of whether a state permit has been issued and of the THC content—are subject to the CSA and must obtain a federal permit: Under the CSA, anyone who seeks to grow marijuana for any purpose must first obtain a DEA registration authorizing such activity. However, several persons have claimed that growing marijuana to produce so-called "hemp" (which purportedly contains a relatively low percentage of THC) is not subject to CSA control and requires no DEA registration. All such claims have thus far failed, as every federal court that has addressed the issue has ruled that any person who seeks to grow any form of marijuana (no matter the THC content or the purpose for which it is grown) must obtain a DEA registration. Regarding states that have enacted laws legalizing cannabis grown for industrial purposes, DEA had stated "these laws conflict with the CSA, which does not differentiate, for control purposes, between marijuana of relatively low THC content and marijuana of greater THC content." Source: CRS from DEA, "DEA History in Depth," 1999-2003, and other DEA published resources. DEA-cited court cases: New Hampshire Hemp Council, Inc. v. Marshall, 203 F.3d I (1st Cir 2000); United States v. White Plume, supra; Monson v. DEA, 522 F.Supp.2d 1188 (D. N.D. 2007), No. 07-3837 (8th Cir. 2007). |
There is limited information about DEA's permit process and on facilities that are licensed to grow hemp, even for research purposes. Previous reports indicate that DEA had issued a permit for an experimental quarter-acre plot at the Hawaii Industrial Hemp Research Program from 1999 to 2003 (now expired).75 Most reports indicate that DEA was reluctant to grant licenses to grow hemp, even for research purposes.76 Some land grant university researchers have been granted licenses to conduct hemp research under certain conditions.77
Dispute over Hemp Imports (1999-2004)
Starting in late 1999, DEA acted administratively to demand that the U.S. Customs Service enforce a zero-tolerance standard for the THC content of all forms of imported hemp—and hemp foods in particular. Development of DEA's rules to support its actions sparked a fierce battle over the permissibility of imported hemp-based food products that lasted from 1999 until 2004.
DEA followed up, in October 2001, with publication of an interpretive rule in the Federal Register explaining the basis of its zero-tolerance standard.78 It held that when Congress wrote the statutory definition of marijuana in 1937, it "exempted certain portions of the Cannabis plant from the definition of marijuana based on the assumption (now refuted) that such portions of the plant contain none of the psychoactive component now known as THC."
In March 2003, DEA issued two final rules addressing the legal status of hemp products derived from the cannabis plant. It found that hemp products "often contain the hallucinogenic substance tetrahydrocannabinols (THC) ... the primary psychoactive chemical found in the cannabis (marijuana) plant."79 Although DEA acknowledged that "in some cases, a Schedule I controlled substance may have a legitimate industrial use," such use would be allowed only under highly controlled circumstances. These rules set forth what products may contain "hemp" and also prohibit "cannabis products containing THC that are intended or used for human consumption (foods and beverages)."
Both the proposed rule (which was published concurrently with the interpretive rule) and the final 2003 rule gave retailers of hemp foods a date after which DEA could seize all such products remaining on shelves. On both rules, hemp trade associations requested and received court-ordered stays blocking enforcement of that provision. DEA's interpretation made hemp with any THC content subject to enforcement as a controlled substance.
Hemp industry trade groups, retailers, and a major Canadian exporter filed suit against DEA, arguing that congressional intent was to exempt plant parts containing naturally occurring THC at non-psychoactive levels, the same way it exempts poppy seeds containing trace amounts of naturally occurring opiates.80 Industry groups maintain that (1) naturally occurring THC in the leaves and flowers of cannabis varieties grown for fiber and food is already at below-psychoactive levels (compared with drug varieties); (2) the parts used for food purposes (seeds and oil) contain even less; and (3) after processing, the THC content is at or close to zero. U.S. and Canadian hemp seed and food manufacturers have in place a voluntary program for certifying low, industry-determined standards in hemp-containing foods. Background information on the TestPledge Program is available at http://www.TestPledge.com. The intent of the program is to assure that consumption of hemp foods will not interfere with workplace drug testing programs or produce undesirable mental or physical health effects.
On February 6, 2004, the U.S. Court of Appeals for the Ninth Circuit permanently enjoined the enforcement of the final rule.81 The court stated that "DEA's definition of 'THC' contravenes the unambiguously expressed intent of Congress in the CSA and cannot be upheld."82 In late September 2004 the Bush Administration let the final deadline pass without filing an appeal.83
In January 2017, HIA petitioned the U.S. Court of Appeals for the Ninth Circuit to block DEA's implementation of its December final rule on marijuana extracts, which would designate certain hemp-derived nonpsychotropic products, such as CBD, as a "marihuana extract" subject to the CSA.84 Then, in February, 2017, HIA again petitioned the court alleging that DEA violated the court's 2004 order when it indicated that a North Dakota hemp company would need a DEA registration and would be subject to other requirements before it could ship processed hemp products outside the state, even though these products were in accordance with state law and the 2014 farm bill.85
In May 2018, DEA issued an internal directive to further clarify the ruling in the 2004 court case.86 The directive acknowledges that products and materials made from the cannabis plant that fall outside the CSA's definition of marihuana—such as sterilized seeds incapable of germination, oil or cake made from the seeds, mature stalks, and fiber from mature stalks—are exempt from CSA and may be "sold and otherwise distributed throughout the United States without restriction under the CSA or its implementing regulations."87 Exempt cannabis plant material also includes "any other compound, manufacture, salt, derivative, mixture, or preparation" of the above items, despite the presence of cannabinoids. The directive further acknowledges that such exempt products and materials may be imported into the United States without restriction (under the Controlled Substances Import and Export Act, 21 U.S.C. §§951-971) or exported from the United States ("provided further that it is lawful to import such products under the laws of the country of destination"). The directive does not address marijuana extracts and resins.
Some in the hemp industry are interpreting the 2018 directive as providing an indication of DEA's position regarding extracts such as CBD from exempt plant materials, including industrial hemp. They claim that this could provide an indication that CBD extracted from hemp could be considered exempt from CSA regulation and DEA's jurisdiction.88 They also acknowledge that some research indicates that meaningful levels of CBD might not be readily extracted from exempt plant materials such as industrial hemp.
2013 DEA Guidance Outlined in "Cole Memo"
In August 2013, the Department of Justice (DOJ) updated its federal marijuana enforcement policy following 2012 state ballot initiatives in Washington and Colorado that "legalized, under state law, the possession of small amounts of marijuana and provide for the regulation of marijuana production, processing, and sale."89 The guidance—commonly referred to as the "Cole memo"—outlines DOJ's policy, clarifying that "marijuana remains an illegal drug under the Controlled Substances Act and that federal prosecutors will continue to aggressively enforce this statute." DOJ identified eight enforcement areas that federal prosecutors should prioritize:
- 1. Preventing the distribution of marijuana to minors,
- 2. Preventing revenue from the sale of marijuana from going to criminal enterprises, gangs, and cartels,
- 3. Preventing the diversion of marijuana from states where it is legal under state law in some form to other states,
- 4. Preventing state-authorized marijuana activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity,
- 5. Preventing violence and the use of firearms in the cultivation and distribution of marijuana,
- 6. Preventing drugged driving and the exacerbation of other adverse public health consequences associated with marijuana use,
- 7. Preventing the growing of marijuana on public lands and the attendant public safety and environmental dangers posed by marijuana production on public lands, and
- 8. Preventing marijuana possession or use on federal property.
Although the Cole memo does not specifically address industrial hemp, because DOJ regards all varieties of the cannabis plant as "marijuana" and does not distinguish between low- and high-THC varieties, the August 2013 guidance appears to cover industrial hemp production as well. Accordingly, some are interpreting the guidance as allowing states to proceed to implement their laws regulating and authorizing the cultivation of hemp.90
Changes to Colorado's state laws in November 2012 now allow for industrial hemp cultivation. Industrial hemp was reported as being grown in Colorado in 2013.91 However, growers and state authorities continue to face a number of challenges implementing Colorado's law, including sampling, registration and inspection, seed availability and sourcing, disposition of non-complying plants, and law enforcement concerns, as well as production issues such as hemp agronomics, costly equipment, and limited manufacturing capacity, among other grower and processor concerns.92 There is also general uncertainty about how federal authorities will respond to production in states where state laws allow cultivation.
In November 2012, state authorities in Colorado requested clarification from DOJ about how federal enforcement authorities might respond to its newly enacted laws and forthcoming regulations.93 Since federal law regards all varieties of the cannabis plant as "marijuana," many continue to regard DOJ's August 2013 guidance as also likely applicable to the regulation of industrial hemp.94 In November 2013, Colorado officials requested further clarification regarding the cultivation of industrial hemp specifically.95 It is not known whether either federal agency has responded to the state's requests.
In September 2013, Representative Blumenauer sent a letter to Oregon state officials urging them to implement that state's hemp laws.96 In response, DOJ officials in Oregon reiterated that since "'industrial hemp' is marijuana, under the CSA, these eight enforcement priorities apply to hemp just as they do for all forms of cannabis" and that "federal prosecutors will remain aggressive" when it comes to protecting these eight priorities.97 They further indicated that they do not intend to interfere with their state's hemp production so long as it is well-regulated and subject to enforcement.98 Some regard that correspondence as indicative of how federal authorities might respond to production in states that permit growing and cultivating hemp.99
In January 2018, Attorney General Jeff Sessions sent a memorandum to all U.S. Attorneys rescinding previous nationwide guidance specific to marijuana enforcement, including the 2013 Cole Memo.100 Since both the Cole Memo and the 2018 Sessions memorandum are focused on marijuana enforcement, some maintain that this action does not impact ongoing industrial hemp efforts in some states.101
DEA's Blocking of Imported Viable Hemp Seeds
In response to the enactment of the 2014 farm bill provision allowing for the cultivation of industrial hemp by research institutions and state departments of agriculture, several states made immediate plans to initiate new hemp pilot projects.
Kentucky announced plans for several pilot projects through the Kentucky Department of Agriculture. However, in May 2014, U.S. Customs officials blocked the department's shipment of 250 pounds of imported viable hemp seed from Italy at Louisville International Airport. DEA officials contend that the action was warranted since the "importation of cannabis seeds continues to be subject to the Controlled Substances Import and Export Act (CSIEA)"102 and to the implementing regulations, which restrict persons from importing viable cannabis seed unless they are registered with DEA and have obtained the necessary Schedule I research permit, among other requirements.
Viable seeds are seeds that are alive and have the potential to germinate and develop into normal reproductively mature plants, under appropriate growing conditions. DEA has required that seeds be either heat sterilized or steam sterilized to remove any naturally occurring traces of THC, which makes the seeds mostly incapable of germination. DEA regulates the importation, sterilization, and commercial distribution of hemp seed pursuant to CSIEA.103
To facilitate release of the hemp seeds, the Kentucky Department of Agriculture filed a lawsuit in U.S. District Court against DEA, DOJ, U.S. Customs and Border Protection, and the U.S. Attorney General.104 In the lawsuit, the department contends that its efforts to grow industrial hemp are authorized under both state and federal law and that DEA should not seek to impose "additional requirements, restrictions, and prohibitions" on hemp production beyond requirements in the 2014 farm bill or otherwise interfere with its delivery of hemp seeds.
Kentucky's seeds were eventually released and planted. However, these actions resulted in uncertainty for U.S. hemp growers. Some in the industry claim that DEA continues to initiate policy changes intended to block hemp cultivation.105 In response, Congress enacted additional legislation to stop DEA from intervening in the implementation of the 2014 farm bill provision. (For more information, see "Selected Appropriations Actions".)
Although hemp production is now allowed in accordance with the requirements under the 2014 farm bill provision, the importation of viable seeds still requires DEA registration according to CSIEA (21 U.S.C. §§951-971). This requirement was reinforced in a 2016 joint "Statement of Principles" on industrial hemp from DEA, USDA, and FDA.106 Purchasing viable seed for germination continues to be a complicated process. It can be difficult to locate a seed source, since there are no U.S. cultivars, and any seed must be sourced internationally. Also, the grower must submit a DEA 357 import form, and any seed source must be pre-screened by DEA and also meet USDA phytosanitary rules. Once the permit is obtained, a copy of the permit is then sent to the seed supplier and may be shipped by air freight.107 Other requirements include approval for entry and ground transport to field sites and field site security.
2016 Joint "Statement of Principles" on Industrial Hemp
In August 2016, DEA issued three major decisions on marijuana and industrial hemp.108 Regarding marijuana, DEA announced it was rejecting a petition to reschedule marijuana (affirming its continued status as an illegal Schedule I controlled substance).109 It also announced certain policy changes regarding authorized marijuana cultivators for research.110 Regarding industrial hemp, DEA issued a joint statement with USDA and FDA on the principles on industrial hemp.
The three federal agencies acknowledged that the 2014 farm bill provision regarding industrial hemp "left open many questions regarding the continuing application of Federal drug control statutes to the growth, cultivation, manufacture, and distribution of industrial hemp products, as well as the extent to which growth by private parties and sale of industrial hemp products are permissible."111 The 2014 farm bill also "did not remove industrial hemp from the controlled substances list." Federal law continues to restrict hemp-related activities that were not specifically legalized under the farm bill provision, which did not amend CSA requirements regarding the manufacture and distribution of "drug products" containing controlled substances. The farm bill provision also did not amend the Federal Food, Drug, and Cosmetic Act112 regarding the approval process for new drug applications.
The joint statement restates the 2014 farm bill's requirement that hemp be grown and cultivated "in accordance with an agricultural pilot program ... established by a State department of agriculture or State agency ... in a State where the production of industrial hemp is otherwise legal under State law."113 It further notes that "state registration and certification of sites used for growing or cultivating industrial hemp" were not addressed in the 2014 farm bill and recommends that "such registration should include the name of the authorized manufacturer, the period of licensure or other time period during which such person is authorized by the State to manufacture industrial hemp, and the location, including Global Positioning System coordinates, where such person is authorized to manufacture industrial hemp."
Among the noted positive aspects of the joint statement is clarification by the federal agencies about who is able to grow or cultivate industrial hemp as part of a state's agricultural research pilot program and the applicability of USDA research and other programs to support industrial hemp. Other aspects of the joint statement, however, have raised concerns regarding how the federal agencies view the statutory definition of industrial hemp and also possible restrictions on the sale of industrial hemp products and the importation of viable seeds for growing and cultivation. Each of these is discussed in the following sections.
Many in Congress and in the hemp industry had much anticipated clarification regarding DEA's position on industrial hemp, given continued uncertainty and despite support for hemp cultivation in the 2014 farm bill. The joint statement provides guidance to "individuals, institutions, and states" on a number of issues pertaining to the growing and cultivation of hemp. While some in Congress and in the industry are encouraged by parts of the joint statement, they have expressed concerns about other aspects of the joint statement.114 A summary of these issues is as follows.
- Clarification regarding who can grow/cultivate hemp. The joint statement acknowledges that the 2014 farm bill authorized "State departments of agriculture, and persons licensed, registered, or otherwise authorized by them" and "institutions of higher education or persons employed by or under a production contract or lease with them" to grow or cultivate industrial hemp as part of an agricultural pilot program in accordance with the 2014 farm bill. This seemingly clears up confusion regarding the potential participation of private farmers licensed or under contract with authorized state departments of agriculture and institutions of higher learning.
- Clarification regarding USDA research support for hemp. The joint statement clarifies that institutions of higher education and other authorized participants "may be able to participate in USDA research or other programs to the extent otherwise eligible for participation in those programs." This seemingly addresses questions raised in November 2015 by some Members of Congress as part of a letter sent to USDA requesting clarification on the extent to which federal funds may be used to support research on industrial hemp.
- Confusion regarding the definition of industrial hemp. Some in the hemp industry worry that the joint statement reinterprets the statutory definition of industrial hemp to cover fiber and seed only, excluding flowering tops, which they believe is covered by the farm bill definition.115 The flowering heads of the plant have the greatest cannabinoid content. They also worry that the joint statement expands upon inherent restrictions to the statutory definition in that it broadly highlights the term THC, which is defined to include "all isomers, acids, salts, and salts of isomers of tetrahydrocannabinols," whereas the statutory definition in the 2014 farm bill specifies delta-9 THC, the dominant psychoactive cannabinoid of cannabis. Some in Congress claim that the executive branch is defining industrial hemp more narrowly than that defined in statute in that it "drops the 'delta-9' when describing tetrahydrocannabinol" and "adds isomers, acids, and salts of isomers of THC to count against the 0.3% THC threshold."116 These Members of Congress have asked that the definition be removed from the guidance.
- Confusion regarding possible restrictions on commerce. Some in Congress note that the 2014 farm bill defined ''agricultural pilot program'' to mean "a pilot program to study the growth, cultivation, or marketing of industrial hemp" (italics added).117 These Members of Congress have asked for confirmation that "general commercial activity" does not prevent any types of sale from occurring from the framework of an approved pilot program. Likewise, the hemp industry remains concerned about the inclusion of language in the joint statement indicating that "industrial hemp products ... may not be sold in States where such sale is prohibited."118 Broadly speaking "industrial hemp products" are already widely marketed, sold, and distributed. Some claim that this restriction on sales is contrary to provisions in both the CSA and the 2014 farm bill.
- Confusion regarding the transportation and sales of hemp. The joint statement also emphasizes that "industrial hemp plants and seeds may not be transported across State lines," and restates DEA's position that the importation of viable cannabis seeds be carried out by DEA-registered persons, in accordance with CSIEA, seemingly to limit the sale of hemp products only in states with industrial hemp pilot programs. This remains a contentious issue following DEA's blocking of viable hemp seed in 2014. Some in Congress maintain that federal agencies do not have the authority to limit hemp sales or prohibit the transport of plants or seed under the 2014 farm bill.119
The joint statement's guiding principles are provided in the Appendix B.
Additional confusion remains, however, since the joint statement explicitly says it "does not establish any binding legal requirements," further raising questions about whether guidance in the statement could influence future DEA policies and enforcement action regarding industrial hemp cultivation and marketing.
2018 Restrictions on SBA Loans
In April 2018, the Small Business Administration (SBA) prohibited banks from issuing SBA-backed loans to any "business that grows, produces, processes, distributes or sells products purportedly made from 'hemp' … unless the business can demonstrate that its business activities and products are legal under federal and state law. Examples of legal hemp products include paper, clothing and rope." Given the continued uncertainty about the legality of the marketing of industrial hemp products, it may be difficult for SBA to determine if a business's activities and products are legal under federal law, which could restrict hemp businesses from obtaining SBA-backed loans.
Other Federal Agency Actions
In 1994, President Clinton issued Executive Order 12919, "National Defense Industrial Resources Preparedness," which was intended to strengthen the U.S. industrial and technology base for meeting national defense requirements. The order included hemp among the essential agricultural products that should be stocked for defense preparedness purposes.120 Some hemp supporters have argued that the executive order gives hemp a renewed value as a strategic crop for national security purposes in line with its role in World War II.121
USDA has supported research on alternative crops and industrial uses of common commodities since the late 1930s. Some alternative crops have become established in certain parts of the United States—kenaf (for fiber) in Texas, jojoba (for oil) in Arizona and California, and amaranth (for nutritious grain) in the Great Plains states. Many have benefits similar to those ascribed to hemp but are not complicated by having a psychotropic variety within the same species.
The Critical Agricultural Materials Act of 1984 (P.L. 98-284, 7 U.S.C. §178) supports the supplemental and alternative crops provisions of the 1985 and 1990 omnibus farm acts and other authorities and funds research and development on alternative crops at USDA and state laboratories.122 In addition, Section 1473D of the National Agricultural Research, Extension, and Teaching Policy Act of 1977 (7 U.S.C. §3319d(c)) authorizes USDA to make competitive grants toward the development of new commercial products derived from natural plant material for industrial, medical, and agricultural applications. To date, these authorities have not been used to develop hemp cultivation and use.
The United States is a signatory of the United Nations Single Convention on Narcotic Drugs, 1961.123 The principal objectives of the convention are to "limit the possession, use, trade in, distribution, import, export, manufacture and production of drugs exclusively to medical and scientific purposes and to address drug trafficking through international cooperation to deter and discourage drug traffickers."124 The convention requires that each party control cannabis cultivation within its borders. However, Article 28.2 of the convention states, "This Convention shall not apply to the cultivation of the cannabis plant exclusively for industrial purposes (fibre and seed) or horticultural purposes." Thus the convention need not present an impediment to the development of a regulated hemp farming sector in the United States.
Ongoing Congressional Activity
2018 Farm Bill Debate
Congress has continued to introduce legislation to further advance industrial hemp and address continued perceived obstacles to hemp production in the United States. Specifically, an expanded version of the Industrial Hemp Farming Act—first introduced in the 109th Congress—was introduced in the 115th Congress in both the House and Senate (H.R. 5485; S. 2667). These bills are further discussed in "Industrial Hemp Farming Act". Many of the provisions in these bills are included in the Senate version of the 2018 farm bill legislation (S. 3042) that is now being debated in Congress.
House Farm Bill (H.R. 2)
A number of hemp-related amendments to the House Agriculture Committee bill (Agriculture and Nutrition Act of 2018, H.R. 2) were proposed and/or considered but not adopted.
During House committee markup, Representative Comer considered but did not propose an amendment to H.R. 2 that would clarify that federally recognized Indian tribes are eligible to grow hemp in accordance with the conditions specified in the 2014 farm bill.125 It would have also required USDA to develop guidance on standardized testing procedures for the THC concentration for industrial hemp.
Amendments regarding hemp were also submitted for consideration by the House Rules Committee but were not made in order and so were not allowed to proceed during the House floor debate on H.R. 2. One bipartisan proposal submitted by Representatives Massie and Polis proposed to remove industrial hemp from the CSA definition of marihuana. Another proposal submitted by Representatives Comer and Blumenauer, among others, also proposed to remove industrial hemp from the CSA definition and place hemp in the jurisdiction of the USDA as an agricultural commodity. Another amendment proposed by Representative Barr would create a safe harbor for financial institutions that provide services to hemp businesses authorized under the 2014 farm bill. None of these amendments or other provisions regarding industrial hemp are included in H.R. 2.
Senate Farm Bill (S. 3042)
The Senate Agriculture, Nutrition, and Forestry Committee farm bill (Agriculture Improvement Act of 2018, S. 3042) includes a number of provisions regarding industrial hemp within the bill's Horticulture title, Research title, Crop Insurance title, and Miscellaneous title (Appendix C). Many of these provisions originated in the Industrial Hemp Farming Act of 2018 (S. 2667; H.R. 548).126
Chief among these is a provision that would amend the CSA to exclude industrial hemp as it is defined in the 2014 farm bill (i.e., as containing no more than a 0.3% THC concentration) from the statutory definition of marihuana.127 The Senate farm bill also creates a new hemp program under the Agricultural Marketing Act of 1946 (7 U.S.C. §1621 et seq.), expanding the existing statutory definition of hemp and expanding eligibility to other producers and groups, including tribes and territories. States or Indian tribes wanting primary regulatory authority over hemp production would be required to implement a "plan" to further monitor and regulate hemp production. Other provisions in the Crop Insurance title would make hemp producers eligible to participate in federal crop insurance programs, while provisions in the Research title of the bill would make hemp production eligible for certain USDA research and development programs.
Industrial Hemp Farming Act
The Industrial Hemp Farming Act of 2018 (Comer/H.R. 5485; McConnell/S. 2667) is intended to facilitate the possible commercial cultivation of industrial hemp in the United States. The bills would amend Section 102 of the CSA (21 U.S.C. 802(16)) to exclude "industrial hemp" from the statutory definition of marihuana. Industrial hemp would be defined based on its THC content and set at a threshold of 0.3% THC. Such a change could remove low-THC hemp from being covered by the CSA as a controlled substance subject to DEA regulation, thus allowing for industrial hemp to be grown and processed under some state laws. The bill could grant authority to any state permitting industrial hemp production and processing to determine whether any such cannabis plants met the limit on THC concentration as set forth in the CSA. In any criminal or civil action or administrative proceeding, the state's determination may be conclusive and binding.
H.R. 5485 and S. 2667 would repeal the hemp pilot program established in the 2014 farm bill and replace it with a new program as part of a new "Hemp Production" subtitle under the Agricultural Marketing Act of 1946 (7 U.S.C. §1621 et seq.). The new program expands upon the existing statutory definition to include any part of the Cannabis plant, including "the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing, or not."128 It would clarify that allowable cultivation includes (in addition to states) tribal governments,129 the District of Columbia, the Commonwealth of Puerto Rico, and any U.S. territory or possession. Eligibility of "state department of agriculture" would be amended to mean the "agency, commission, or department of a state government responsible for agriculture in the state." State or Indian tribes wanting primary regulatory authority over hemp production would be required to implement a "plan" under which the state or Indian tribe monitor and regulate hemp production. State and tribal plans would require grower information collection and procedures for testing, disposal (of hemp grown in violation and the law), and compliance. H.R. 5485 and S. 2667 authorize appropriations ("such sums as are necessary") for USDA to support and enforce state and tribal plans and further specifies requirements regarding the plan approval process, USDA technical assistance to develop plans, and necessary corrective action for plan violations.130
H.R. 5485 and S. 2667 further address industrial hemp as part of the federal crop insurance program and include hemp as eligible for research funding under the Supplemental and Alternative Crops Act131 and the Critical Agricultural Materials Act,132 which are authorized to receive $1 million in annual appropriations through FY2018. Finally, the bills require that USDA conduct a study of USDA agricultural pilot programs, including the hemp pilot program, which would be repealed one year after enactment. USDA would also be required to conduct a study of USDA agricultural pilot programs, including the hemp pilot program in the 2014 farm bill.
Earlier in the 115th Congress, Representative Comer introduced a different version of the bill as part of the Industrial Hemp Farming Act of 2017 (H.R.3530). In addition to exempting industrial hemp from definitions of marihuana in CSA, this version of the bill proposed to further expand the statutory definition of hemp to include viable seeds and to clarify that allowable cultivation includes Native American tribes133 in addition to states. It also includes a new definition for research hemp to mean any part or derivative of the Cannabis plant (including viable seeds) that has a delta-9 THC concentration of more than 0.3% on a dry weight basis but less than 0.6% on a dry weight basis and that is used in scientific, medical, or industrial research conducted by an institution of higher education or a state department of agriculture. H.R. 3530 would also require that states and tribes, upon the request of the U.S. Attorney General, submit information regarding hemp production, storage, distribution, or use.134
Each of these versions of the Industrial Hemp Farming Act greatly expand upon previous versions of the bill. The Industrial Hemp Farming Act was first introduced in the 109th Congress by former Representative Ron Paul and was reintroduced in subsequent legislative sessions (H.R. 1831, 112th Congress; H.R. 1866, 111th Congress; H.R. 1009, 110th Congress; H.R. 3037, 109th Congress). In the 112th Congress, Senator Ron Wyden introduced S. 3501 in the Senate.135 Representative Massie and Senator Wyden also introduced versions of these same bills in the 113th and 114th Congresses.136 Some in Congress believe that industrial hemp production could result in economic and employment gains in some states and regions.137
Legislation Regarding Possible Medical Applications of Hemp
Legislation introduced in both the House and Senate has addressed the potential therapeutic uses of industrial hemp to allow for its production and use as CBD. CBD is a non-psychoactive compound in Cannabis that is low in delta-9 THC.138 CBD is sold as an extract and marketed as helping to address various ailments, including neuropathic pain, epilepsy, post-traumatic stress disorder, nausea as a result of chemotherapy, and other disorders. Most CBD extracts currently being marketed for certain therapeutic purposes are generally formulated from strains of medical cannabis with THC levels higher than 0.3% but generally less than 1% THC.139 Some hemp-based CBD products—mostly dietary supplements—have been marketed as being rich in CBD and as having comparable therapeutic uses to CBD extracts. Fraudulent marketing claims by some hemp-based CBD products have resulted in the FDA issuing a series of warning letters to several companies since 2015.140
In the 115th Congress, the Therapeutic Hemp Medical Access Act of 2017 (S. 1008) and the Charlotte's Web Medical Access Act of 2017 (H.R. 2273)141 would amend CSA by excluding cannabidiol and cannabidiol-rich plants, defined as having a delta-9 THC concentration of no more than 0.3% on a dry weight basis. Similar versions of these bills were introduced in the 114th Congress and 113th Congress.142 The House and Senate bills are related but are not identical. In addition to removing cannabidiol and cannabidiol-rich plants, as defined, from regulation under CSA, the House bill would further exclude these from being applicable to requirements under the Federal Food, Drug, and Cosmetic Act, which broadly regulates the quality and safety of foods and dietary supplements. This provision is not part of the Senate bill.
There is also growing concern that hemp-based CBD products, derived from industrial hemp, are being marketed as being rich in CBD and as having comparable therapeutic uses to CBD extracts. Medicine-grade CBD is not produced or pressed from hemp seeds. Hemp seed oil, marketed as "hemp oil," is made by pressing hemp seeds that contain low levels of CBD (typically less than 25 parts per million). Most of the CBD extracts currently being marketed for certain therapeutic purposes are generally formulated from strains of cannabis with THC levels higher than 0.3% but generally less than 1% THC.143 Some claim that scientific research shows that meaningful levels of CBD cannot be extracted from hemp.144 Also, FDA has continued to issue a number of notices and warning letters regarding its concerns about CBD, which is being marketed across a range of therapeutic/medicinal products.145 For more information, see CRS In Focus IF10391, Potential Use of Industrial Hemp in Cannabidiol Products.
To date, FDA has not approved any drug product containing CBD for any indication and has issued warning letters to several companies that market CBD products to treat health conditions for both humans and pets. According to FDA, these products are not "generally recognized as safe and effective," and the companies marketing these products are engaging in illegal interstate commerce.146 FDA has further determined that products containing CBD cannot be sold as dietary supplements and are excluded from the dietary supplement definition in the Federal Food, Drug, and Cosmetic Act.147 As such, FDA may consult with its federal and state partners about whether to initiate a federal enforcement action against the manufacturers of CBD products that are marketed as dietary supplements.148 In June 2015, the Senate Caucus on International Narcotics Control held a hearing on the barriers to research and the potential medical benefits of CBD. (Additional information is provided in the text box below.)
|
Senate Caucus on International Narcotics Control (June 2015 Hearing) In June 2015, the Senate Caucus on International Narcotics Control, led by Senators Chuck Grassley and Dianne Feinstein, held a hearing on the barriers to research and the potential medical benefits of CBD. The caucus leaders claimed many leading medical organizations have called for further research into the potential medical use of CBD. The hearing addressed the complexities involved with conducting CBD research, as well as its potential medical benefits and risks in treating serious illnesses. The hearing provided a follow-up to letters sent by the caucus leaders to DOJ and to the Department of Health and Human Services (HHS) to ask these agencies to evaluate CBD using the appropriate scientific and medical factors to make a scheduling determination for it that is separate from the whole marijuana plant. The caucus anticipates that "[i]f it turns out that CBD may be classified on a lower schedule than the entire marijuana plant, and then research on it may proceed somewhat more easily." The caucus reported that DOJ and HHS have agreed to undertake this evaluation, representing that "for the first time, the federal government will conduct a comprehensive analysis to determine whether cannabidiol has scientific and medical value." Source: CRS based on opening statement of Senator Chuck Grassley, chairman, Senate Caucus on International Narcotics Control Committee, June 24, 2015; and Senator Dianne Feinstein, "Feinstein, Grassley Announce New Federal Policy on Cannabidiol Research," press release, June 23, 2015. See also letter from DOJ to Senator Feinstein, January 5, 2015; letter from HHS to Senator Grassley, May 13, 2015; and letter from DOJ to Senators Grassley and Feinstein, June 23, 2015. |
Many agriculture-based groups continue to advocate for the need for additional research into the possible benefits and uses of industrial hemp-derived CBD.149 Some states continue to conduct research on the potential uses for industrial hemp-derived CBD.150
The National Academies of Sciences, Engineering, and Medicine (NASEM) has broadly reviewed this issue. In February 2017, NASEM published a comprehensive review of existing cannabis research that provides a broad set of evidence-based research conclusions on the health effects of cannabis and cannabinoids and provides recommendations to support advancing future research and inform public health decisions.151 It claims that there is conclusive or substantial evidence that oral cannabinoids are effective antiemetics in the treatment of chemotherapy-induced nausea and vomiting and for improving patient-reported multiple sclerosis spasticity symptoms.152 Others have also documented possible medical uses of cannabis.153 The study, however, does not distinguish between cannabinoids from low and high THC strains or between hemp-derived cannabinoids and cannabinoids from other cannabis strains.
Other Introduced Legislation
A number of other bills regarding industrial hemp have been introduced in the 115th Congress. The Industrial Hemp Banking Act (H.R. 4711) would identify hemp production as a legitimate business. It would similarly exempt hemp production from CSA's definition of marihuana and would also prohibit regulators from denying banking services to hemp producers.154 In addition, the Industrial Hemp Water Rights Act (H.R. 4164, S.1576) would prohibit regulators from denying hemp growers access to water—regardless of whether the water is part of a federal water project—if the hemp cultivation is authorized under the laws of the state where it is grown.
Congressional Action on USDA Hemp Research Support
In November 2015, several Members of Congress sent a letter to USDA requesting clarification of the agency's research funds for industrial hemp.155 This action was in response to questions by a number of state and private research institutions on the extent to which industrial hemp initiatives were eligible for U.S. federal grant awards (both USDA and non-USDA program funds). These questions arose, in part, given mixed messages received by some land grant universities about whether they would qualify for USDA competitive grants to do industrial hemp research and initial indications that they would be denied such support. Some groups feared they could jeopardize eligibility for other grants if they pursued research into industrial hemp.
In late 2015, CRS staff attempted to get further clarification on USDA's policy regarding industrial hemp and federal grants and loans to support research of industrial hemp with limited success. Information provided from USDA was not always consistent and often conflicting.156 According to USDA's National Institute of Food and Agriculture (NIFA), the agency had not awarded any competitive research grants for industrial hemp (as of September 2015).157 However, subsequent searches of USDA's Current Research Information System (CRIS) database158 indicate that NIFA formula-funded grants were used at Colorado State University for 2015 under available Hatch Act funding to study hemp cultivation as part of bigger grants about profitability of alternative agriculture in southern Colorado.159 Other available information, including correspondence between USDA and various congressional staff, suggests that USDA has no record of any application for industrial hemp research being denied. No additional information is available on whether any such applications had been proposed or would or could be approved.
A USDA memo dating back to December 2014 states that "NIFA supports" grants for industrial hemp research so long as that research meets existing state requirements consistent with the requirements in the 2014 farm bill (P.L. 113-79, §7606; 7 U.S.C. 5940).160 However, USDA staff indicated that the December 2014 memo pertains only to what the statutory provision authorizes and does not say anything explicitly about federal funding of industrial hemp research.161 Although this response did not address the underlying issue regarding federal funding, it likely indicates that researchers working on industrial hemp may carry on with this work at least on their own (according to requirements specified in the 2014 farm bill) without threatening their status and working relationship with USDA.
Other communication with USDA's Rural Development Agency indicated that the agency's Rural Business-Cooperative Service has initiated conversation with USDA's Office of the General Counsel to review whether its programs could potentially support the industrial hemp industry.162 There does not appear to be any legal reason why USDA would not be able to provide grant funding for research activities on industrial hemp within the language of the 2014 farm bill provision, and the question remains about whether USDA will fund such applications in the future. Specifically, clarification is needed regarding whether industrial hemp research projects are eligible for USDA competitive grants (e.g., under USDA's Agriculture and Food Research Initiative program) and/or for Hatch Act formula funds, as well as clarification about whether hemp producers are eligible for other types of agricultural support from other USDA agencies (such as loans and grants administered by USDA's Rural Development Agency).
Some have suggested that perhaps industrial hemp might qualify under certain other USDA grant programs, such as NIFA's Specialty Crop Research Initiative or USDA's Specialty Crop Block Grant Program. However, industrial hemp is not included among the crops that are considered "specialty crops" and technically would not qualify for any grant specifically designated for specialty crop producers.163 Other potential programs include the Organic Transitions Integrated Research Program (ORG) and the Value-Added Producer Grant Program.164
Some constituent groups have also expressed an interest in applying for other non-USDA grants, such as the Small Business Innovation Research program (SBIR) intended to help certain small businesses conduct research and development and is coordinated by the Small Business Administration. CRS has not contacted other federal agencies aside from USDA.
Some of the questions raised by Congress's November 2015 letter were addressed in the 2016 joint statement, but some questions remain, which were again posed in a follow-up letter by several Members of Congress.165 (For additional discussion, see "2016 Joint "Statement of Principles" on Industrial Hemp".)
Groups Supporting/Opposing Further Legislation
In addition to industry groups as well as various state commissions and organizations that are actively promoting reintroducing hemp as a commodity crop in the United States, some key agricultural groups also support U.S. policy changes regarding industrial hemp. For example:
- In 2018, the National Association of State Departments of Agriculture (NASDA) sent a letter to Senate Majority Leader Mitch McConnell and Representative James Comer in support of the Hemp Farming Act of 2018 (S. 2667/H.R. 5485). NASDA claims that the bill addresses "numerous issues hindering the success of industrial hemp pilot programs allowed under the 2014 farm bill."166
- In 2017, the Wisconsin Farm Bureau Federation sent a letter to USDA Secretary Sonny Perdue recommending that the Trump Administration consider hemp to be an agricultural crop. A reported 27 other Farm Bureau presidents supported the initiative.167
- The bipartisan Congressional Cannabis Caucus—launched in February 2017 by Representatives Dana Rohrabacher, Don Young, Earl Blumenauer, and Jared Polis—is focused on policy reforms regarding federal drugs laws and issues regarding legalization in some states.
- The National Farmers Union (NFU) updated its 2013 farm policy regarding hemp to urge the President, Attorney General, and Congress to direct DEA to "reclassify industrial hemp as a non-controlled substance and adopt policy to allow American farmers to grow industrial hemp under state law without affecting eligibility for USDA benefits."168 Previously NFU's policy advocated that DEA "differentiate between industrial hemp and marijuana and adopt policy to allow American farmers to grow industrial hemp under state law without requiring DEA licenses."169
- In 2010, NASDA stated it "supports revisions to the federal rules and regulations authorizing commercial production of industrial hemp" and has urged USDA, DEA, and the Office of National Drug Control Policy to "collaboratively develop and adopt an official definition of industrial hemp that comports with definitions currently used by countries producing hemp." NASDA also "urges Congress to statutorily distinguish between industrial hemp and marijuana and to direct DEA to revise its policies to allow USDA to establish a regulatory program that allows the development of domestic industrial hemp production by American farmers and manufacturers."170 NASDA first adopted a policy on industrial hemp in 2002.
- In 2014, the American Farm Bureau Federation, from efforts led by the Indiana Farm Bureau, endorsed a policy to support the "production, processing, commercialization, and utilization of industrial hemp"171 and reportedly also passed a policy resolution to oppose the "classification of industrial hemp as a controlled substance." Previously, in 1995, the Farm Bureau had passed a resolution supporting "research into the viability and economic potential of industrial hemp production in the United States ... [and] further recommend that such research includes planting test plots in the United States using modern agricultural techniques."172
- Regional farmers' organizations also have policies regarding hemp. For example, the North Dakota Farmers Union, as part of its federal agricultural policy recommendations, has urged "Congress to legalize the production of industrial hemp."173 The Rocky Mountain Farmers Union has urged "Congress and the USDA to re-commit and fully fund research into alternative crops and uses for crops" including industrial hemp. Also, they "support the decoupling of industrial hemp from the definition of marijuana" under the CSA and "demand the President and the Attorney General direct the U.S. Drug Enforcement Agency (DEA) to differentiate between industrial hemp and marijuana and adopt a policy to allow American farmers to grow industrial hemp under state law without requiring DEA licenses" to "legalize the production of industrial hemp as an alternative crop for agricultural producers."174
- The National Grange voted in 2009 to support "research, production, processing and marketing of industrial hemp as a viable agricultural activity."175
- In California, ongoing efforts to revise the definition of marijuana to exclude "industrial hemp" (SB 566) are supported by the state's sheriffs' association.176 The county farm bureau and two sheriffs' offices supported previous efforts in 2011 to establish a pilot program to grow industrial hemp in selected counties (although the state's governor later vetoed the bill, SB 676).177
- North American Industrial Hemp Council—a coalition of farmers, state legislators, former officials, scientists, merchants, entrepreneurs, and environmentalists—filed a petition in June 2016 asking DEA to "remove industrial hemp from the federal drug schedules."178
Despite support by some, other groups continue to oppose policy changes regarding cannabis. For example, the National Alliance for Health and Safety, as part of Drug Watch International, claims that proposals to reintroduce hemp as an agricultural crop are merely a strategy by "the international pro-drug lobby to legalize cannabis and other illicit substances."179 The California Narcotic Officers' Association claims that allowing for industrial hemp production would undermine state and federal enforcement efforts to regulate marijuana production, since, they claim, the two crops are not distinguishable through ground or aerial surveillance but would require costly and time-consuming lab work to be conducted.180 This group also claims that these similarities would create an incentive to use hemp crops to mask illicit marijuana production, since marijuana is such a lucrative cash crop.181 Concerns about the potential linkages to the growing and use of illegal drugs are also expressed by some parent and community organizations, such as the Drug Free America Foundation and PRIDE.182
Given DEA's current policy positions and perceived DEA opposition to changing its current policies because of concerns over how to allow for hemp production without undermining the agency's drug enforcement efforts and regulation of the production and distribution of marijuana, hemp proponents say that further policy changes regarding industrial hemp are likely not forthcoming absent congressional legislative action.
Concluding Remarks
Hemp production in the United States faces a number of obstacles in the foreseeable future, such as U.S. government drug policies and DEA concerns about the ramifications of U.S. commercial hemp production. These concerns are that commercial cultivation could increase the likelihood of covert production of high-THC marijuana, significantly complicating DEA's surveillance and enforcement activities and sending the wrong message to the American public concerning the government's position on drugs. DEA officials and a variety of other observers also express the concern that efforts to legalize hemp—as well as those to legalize medical marijuana—are a front for individuals and organizations whose real aim is to see marijuana decriminalized.
Hemp production in the United States also faces competition from other global suppliers. The world market for hemp products remains relatively small, and China, as the world's largest hemp fiber and seed producer, has had and likely will continue to have major influence on market prices and thus on the year-to-year profits of producers and processors in other countries. Canada's head start in the North American market for hemp seed and oil would also likely affect the profitability of a start-up industry in the United States.
Nevertheless, the U.S. market for hemp-based products has a highly dedicated and growing demand base, as indicated by recent U.S. market and import data for hemp products and ingredients, as well as market trends for some natural foods and body care products. Given the existence of these small-scale, but profitable, niche markets for a wide array of industrial and consumer products, commercial hemp industry in the United States could provide opportunities as an economically viable alternative crop for some U.S. growers.
Appendix A. Listing of Selected Hemp Studies
- A Review of Hemp as a Sustainable Agricultural Commodity, Task Force Report by the University of Washington's Henry M. Jackson School of International Studies, 2018.
- J.H. Cherney and E. Small, "Industrial Hemp in North America: Production, Politics, and Potential," Agronomy, vol. 6, no. 56 (2016).
- L. Lane et al., Industrial Hemp: Legal, Political/Social and Economic Issues Raised Over Time, University of Arkansas, 2016.
- University of Kentucky, Economic Considerations for Growing Industrial Hemp: Implications for Kentucky's Farmers and Agricultural Economy, July 2013.
- C. A. Kolosov, "Regulation of Industrial Hemp Under the Controlled Substances Act" UCLA Law Review, vol. 57, no. 237 (October 2009).
- Manitoba Agriculture, National Industrial Hemp Strategy, March 2008 (prepared for Food and Rural Initiative Agriculture and Agri-Food Canada).
- Reason Foundation, "Illegally Green: Environmental Costs of Hemp Prohibition," Policy Study 367, March 2008, http://www.reason.org/ps367.pdf.
- Agriculture and Agri-Food Canada, Canada's Industrial Hemp Industry, March 2007, http://www.agr.gc.ca/misb/spcrops/sc-cs_e.php?page+hemp-chanvre.
- Maine Agricultural Center, An Assessment of Industrial Hemp Production in Maine, January 2007.
- N. Cherrett et al., "Ecological Footprint and Water Analysis of Cotton, Hemp and Polyester," Stockholm Environment Institute, 2005.
- T. R. Fortenbery and M. Bennett, "Opportunities for Commercial Hemp Production," Applied Economics Perspectives and Policy, vol. 26, no. 1 (2004).
- E. Small and D. Marcus, "Hemp: A New Crop with New Uses for North America," Trends in New Crops and New Uses, 2002.
- T. R. Fortenbery and M. Bennett, "Is Industrial Hemp Worth Further Study in the U.S.? A Survey of the Literature," Staff Paper No. 443, July 2001.
- J. Bowyer, "Industrial Hemp (Cannabis sativa L.) as a Papermaking Raw Material in Minnesota: Technical, Economic and Environmental Considerations," Department of Wood and Paper Science Report Series, May 2001.
- K. Hill, N. Boshard-Blackey, and J. Simson, "Legislative Research Shop: Hemp," University of Vermont, April 2000.
- USDA, Economic Research Service, Industrial Hemp in the United States: Status and Market Potential, AGES001E, January 2000.
- M. J. Cochran, T. E. Windham, and B. Moore, "Feasibility of Industrial Hemp Production in Arkansas," University of Arkansas, SP102000, May 2000.
- D. G. Kraenzel et al., "Industrial Hemp as an Alternative Crop in North Dakota," North Dakota State University, AER 402, July 1998.
- E. C. Thompson et al., Economic Impact of Industrial Hemp in Kentucky, University of Kentucky, July 1998.
- D. T. Ehrensing, Feasibility of Industrial Hemp Production in the United States Pacific Northwest, Oregon State University, SB 681, May 1998.
Appendix B. Joint DEA/USDA/FDA "Statement of Principles on Industrial Hemp"
As noted in the joint DEA/USDA/FDA "Statement of Principles on Industrial Hemp," published August 12, 2016, which is excerpted below:
USDA, having consulted with and received concurrence from the U.S. Drug Enforcement Administration (DEA) and the U.S. Food and Drug Administration (FDA), therefore, is issuing this statement of principles to inform the public regarding how Federal law applies to activities involving industrial hemp so that individuals, institutions, and States that wish to participate in industrial hemp agricultural pilot programs can do so in accordance with Federal law.
The growth and cultivation of industrial hemp may only take place in accordance with an agricultural pilot program to study the growth, cultivation, or marketing of industrial hemp established by a State department of agriculture or State agency responsible for agriculture in a State where the production of industrial hemp is otherwise legal under State law.
The State agricultural pilot program must provide for State registration and certification of sites used for growing or cultivating industrial hemp. Although registration and certification is not further defined, it is recommended that such registration should include the name of the authorized manufacturer, the period of licensure or other time period during which such person is authorized by the State to manufacture industrial hemp, and the location, including Global Positioning System coordinates, where such person is authorized to manufacture industrial hemp.
Only State departments of agriculture, and persons licensed, registered, or otherwise authorized by them to conduct research under an agricultural pilot program in accordance with section 7606, and institutions of higher education (as defined in section 101 of the Higher Education Act of 1965 (20 U.S.C. 1001)), or persons employed by or under a production contract or lease with them to conduct such research, may grow or cultivate industrial hemp as part of the agricultural pilot program.
The term "industrial hemp" includes the plant Cannabis sativa L. and any part or derivative of such plant, including seeds of such plant, whether growing or not, that is used exclusively for industrial purposes (fiber and seed) with a tetrahydrocannabinols concentration of not more than 0.3 percent on a dry weight basis. The term "tetrahydrocannabinols" includes all isomers, acids, salts, and salts of isomers of tetrahydrocannabinols.
For purposes of marketing research by institutions of higher education or State departments of agriculture (including distribution of marketing materials), but not for the purpose of general commercial activity, industrial hemp products may be sold in a State with an agricultural pilot program or among States with agricultural pilot programs but may not be sold in States where such sale is prohibited. Industrial hemp plants and seeds may not be transported across State lines.
Section 7606 specifically authorized certain entities to "grow or cultivate" industrial hemp but did not eliminate the requirement under the Controlled Substances Import and Export Act that the importation of viable cannabis seeds must be carried out by persons registered with the DEA to do so. In addition, any USDA phytosanitary requirements that normally would apply to the importation of plant material will apply to the importation of industrial hemp seed.
Section 7606 did not amend the Federal Food, Drug, and Cosmetic Act. For example, section 7606 did not alter the approval process for new drug applications, the requirements for the conduct of clinical or nonclinical research, the oversight of marketing claims, or any other authorities of the FDA as they are set forth in that Act.
The Federal Government does not construe section 7606 to alter the requirements of the Controlled Substances Act (CSA) that apply to the manufacture, distribution, and dispensing of drug products containing controlled substances. Manufacturers, distributors, dispensers of drug products derived from cannabis plants, as well as those conducting research with such drug products, must continue to adhere to the CSA requirements.
Institutions of higher education and other participants authorized to carry out agricultural pilot programs under section 7606 may be able to participate in USDA research or other programs to the extent otherwise eligible for participation in those programs.
Appendix C. Provisions in H.R. 2 and S. 3042 Addressing Hemp, Compared with Current Law
|
Current Law/Policy |
House Agriculture Committee Reported Bill (H.R. 2) |
Senate Agriculture Committee Reported Bill (S. 3042) |
|
Conforming changes to the Controlled Substances Act (CSA). Schedule I of the CSA (21 U.S.C. §§801 et seq.) includes all cannabis varieties under the term marihuana that is defined to mean "all parts of the plant Cannabis sativa," covering both marijuana and industrial hemp. (21 U.S.C. §802(16)) |
No comparable provision. |
Amends Section 102 of the CSA (21 U.S.C. 802(16)) to exclude industrial hemp from the statutory definition of marijuana. Industrial hemp is defined as containing a delta-9 tetrahydrocannabinol (THC, marijuana's primary psychoactive chemical) concentration of not more than 0.3% on a dry weight basis content. (§12608) |
|
Legitimacy of industrial hemp research. Allows an institution of higher education or state department of agriculture to grow or cultivate industrial hemp for research purposes if allowed under the laws of the state in which the institution is located. Establishes a definition for industrial hemp to mean the plant Cannabis sativa with a delta-9 tetrahydrocannabinol (THC) concentration of not more than 0.3% on a dry weight basis." (7 U.S.C. 5940) |
No comparable provision. |
Creates a new "Hemp Production" subtitle under the Agricultural Marketing Act of 1946 (7 U.S.C. §1621 et seq.). The new program expands upon the existing statutory definition to include any part of the cannabis plant, including "the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing, or not cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis." It clarifies that allowable cultivation includes, in addition to states, tribal governments, the District of Columbia, the Commonwealth of Puerto Rico, and any U.S. territory or possession. Eligibility of "state department of agriculture" would be amended to mean the "agency, commission, or department of a state government responsible for agriculture in the state." State or Indian tribes wanting primary regulatory authority over hemp production would be required to implement a "plan" under which the state or Indian tribe monitor and regulate hemp production. State and tribal plans would require grower information collection, procedures for testing, disposal (of hemp grown in violation and the law), and compliance. Authorize appropriations ("such sums as are necessary") for USDA to support and enforce state and tribal plans and further specifies requirements regarding the plan approval process, USDA technical assistance to develop plans, and necessary corrective action for plan violations. (§10111, §10112) Requires USDA to conduct a study of agricultural pilot program, assessing the economic viability of the domestic production and sale of industrial hemp, and review the hemp pilot program and any other agricultural or academic research relating to industrial hemp. (§7415) |
|
Conforming changes to the CSA. Schedule I of the CSA (21 U.S.C. §§801 et seq.) includes all cannabis varieties under the term marihuana that is defines to mean "all parts of the plant Cannabis sativa," covering both marijuana and industrial hemp. (21 U.S.C. §802(16)) |
No comparable provision. |
Amends Section 102 of the CSA (21 U.S.C. 802(16)) to exclude industrial hemp from the statutory definition of marijuana. Industrial hemp is defined as containing a delta-9 tetrahydrocannabinol (THC, marijuana's primary psychoactive chemical) concentration of not more than 0.3% on a dry weight basis content. (§12608) |
|
Supplemental and alternative crops. Section 1473D of the National Agricultural Research, Extension, and Teaching Policy Act of 1977 authorized appropriations through FY2018 to "develop and implement a research project program for the development of supplemental and alternative crops." Authorizes $1 million in appropriations for each of FY2014-FY2018. (7 U.S.C. 3319d) |
Extends program and funding levels through FY2023. Amends the program to include canola and alternative crops "for agronomic rotational purposes and for use as a habitat for honey bees and other pollinators," among other changes. (§7123) |
Extends program and funding levels through FY2023. Amends the program to include canola and alternative crops "for agronomic rotational purposes and for use as a habitat for honey bees and other pollinators," among other changes. Expands eligibility to industrial hemp. (§7125) |
|
Critical Agricultural Materials Act. Section 5(b)(9) of the act provides for basic and applied research, technology development, and technology transfer. (7 U.S.C. 178c(b)(9)) |
No comparable provision. |
Expands scope of the program to study the economic feasibility of developing native agricultural crops to include industrial hemp. (§7401) |
|
Federal Crop Insurance Program. The federal crop insurance program makes available subsidized crop insurance to producers who purchase a policy to protect against individual farm losses in yield, crop revenue, or whole farm revenue. In general, policies offer a guarantee at the individual farm level or area-wide (e.g., county) level. The producer selects coverage level and absorbs the initial loss through the deductible. The insurance guarantee is based on the expected market price (i.e., no statutory minimum prices as in some farm programs). |
No comparable provisions. |
Amends the Federal Crop Insurance Act (7 U.S.C. 1501 et seq.) to (1) expand eligibility to hemp producers, (2) define hemp in accordance with Section 10111 ("Hemp Production") of the bill, (2) include an insurance period for hemp from which to cover loss in value due to a change in market price, and (3) allows the Federal Crop Insurance Corporation to waive certain viability and marketability requirements related to new policy submissions. (§§11101, 11106, 11112, 11120, 11121) |