Introduction
Obesity is a major public health concern in the United States. Two-thirds of adults and one-third of children are overweight or obese.1 Although many factors contribute to obesity, weight gain is generally the result of an energy imbalance—an excess of calories consumed over calories expended.
High rates of obesity and chronic disease have prompted various state and local nutrition labeling initiatives, in addition to other tactics and strategies. The 1990 Nutrition Labeling and Education Act (NLEA, P.L. 101-535) authorized the Food and Drug Administration (FDA) to require nutrition labeling of most foods and dietary supplements, but it did not require the labeling of food sold in restaurants. Section 4205 of the Patient Protection and Affordable Care Act (ACA, P.L. 111-148) amended the Federal Food, Drug, and Cosmetic Act (FFDCA), establishing nutrition labeling requirements for standard menu items offered for sale in chain restaurants or similar retail food establishments (SRFEs) that have 20 or more locations, that conduct business under the same name regardless of the type of ownership of the locations, and that offer the same menu items for sale. The ACA provision required FDA to promulgate regulations specifying the scope of entities and foods covered by the law, as well as details regarding how the required calorie and nutrition information would be conveyed to consumers. In 2011, FDA proposed two rules delineating nutrition labeling requirements for restaurants and SRFEs, as well as vending machines; both rules were finalized and published in the Federal Register on December 1, 2014.2
Prior to the final rule, some food establishments had already begun voluntarily posting nutrition information. However, variable state and local regulations had resulted in a patchwork of labeling requirements, making compliance more challenging for chain food establishments. Certain groups, such as the National Restaurant Association, expressed support for a federal nutrition labeling standard, stating that it would ease the regulatory burden on national chain restaurants.3
This report discusses the role of nutrition labeling in obesity management and prevention; the research on the effectiveness of restaurant menu calorie labeling; FDA's authority to regulate nutrition labeling; and the FDA's final rules on restaurant menu and vending machine labeling. The report also identifies issues for Congress and flags stakeholders' concerns regarding FDA's final menu labeling rule.
Obesity and Menu Labeling
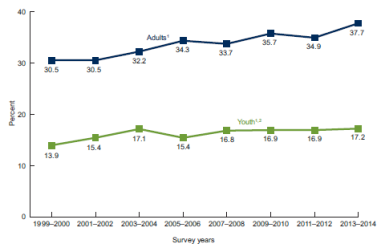
According to the most recent data from the National Health and Nutrition Examination Survey (NHANES), in 2011-2014, the prevalence of obesity in the United States was 36.5% among adults and 17% among youth.4 Although rates appear to have stabilized in youth since 2003-2004 (see Figure 1), the prevalence of obesity among U.S. adults and children is higher than the Healthy People 2020 goals of 30.5% and 14.5%, respectively.5 This is of public health concern because obesity is associated with increased risk of a number of health conditions, such as high blood pressure, type 2 diabetes, cardiovascular disease, and stroke.6
|
Figure 1. Trends in Obesity: United States, 1999-2014 National Health and Nutrition Examination Survey |
 |
|
Source: CDC, Prevalence of Overweight and Obesity Among Adults and Youth: United States, 2011-2014, http://www.cdc.gov/nchs/data/databriefs/db219.htm. Notes: NHANES 2013-2014 is the most recent NHANES data available. Trends in obesity prevalence show no increase among youth since 2003–2004. However, trends do show increases in both adults and youth from 1999–2000 through 2013–2014. There do not appear to be any significant differences in obesity prevalence between 2011–2012 and 2013–2014 in either youth or adults. Youth= ages 2-19 years; Adults= ages 20 years and over. |
Research has shown that frequent eating out is associated with increased calorie intake, and that food eaten away from the home tends to be of lower nutritional quality and higher in saturated fat and sodium than food prepared at home.7 Larger portion sizes served in restaurants contribute to greater calorie intake, and analyses of three fast food chain restaurants indicate that portion sizes have not changed since the mid-1990s (1996-2013).8 Studies also suggest that consumers tend to underestimate the number of calories in restaurant meals.9
Changes in the prevalence of overweight and obesity in the United States have paralleled changes in calorie consumption. As obesity rates increased, calories consumed by individuals two years and older also increased significantly, from a daily average of 1,875 calories in 1977-1978 to 2,002 calories in 2005-2008.10 The same study indicates that in 1977-1978, individuals in the United States consumed approximately 18% of calories away from the home, compared with 32% in 2005-2008.
More recent NHANES data suggest that obesity rates have been leveling off and even decreasing in some age groups (e.g., among two- to five-year-olds).11 Data from USDA's Economic Research Service show that average calorie intake has also declined—by 118 calories (about 5%) between 2005-2006 and 2009-2010. During this time period, consumption of calories from food eaten away from home fell by 127 calories per day, and daily fast food calories fell by 53 calories per day.12 These changes suggest that consumer preferences for nutritious foods may be increasing, and there may be greater use of available nutrition information (e.g., the Nutrition Facts panel), resulting in improved diet quality among consumers.13
Public health stakeholders have generally supported restaurant menu labeling as a policy option for obesity prevention. Proponents of menu labeling say that providing calorie information in restaurants and food establishments may help consumers make healthier and more informed dietary choices.14 However, some researchers have found that current evidence does not support a significant impact of menu labeling on calories ordered,15 suggesting that posting calorie counts does not result in consumers making healthier food choices.16
Research Evaluating the Impact of Menu Labeling
It is difficult to predict what effect, if any, mandatory restaurant menu labeling will have on food purchasing and health outcomes. However, changes in behavior following implementation of calorie labeling regulations in other jurisdictions prior to publication of the final federal rule (e.g., New York City, Philadelphia, and King County, WA) may provide some insight.
Studies of the Impact of Menu Labeling on Calories Purchased
Studies examining the relationship between menu labeling and calorie purchasing behavior have yielded mixed findings. Although consumers often report ordering fewer calories as a result of menu labeling, studies examining restaurant transaction data have not consistently reported a decrease in calories purchased after implementation of menu labeling. This section discusses several studies that have evaluated the impact of menu labeling, using survey and transaction data, on calories purchased.17
Findings from current research are limited because existing studies often vary in scope and methodology.18 For example, several of the studies that did not find a post-labeling decrease in calories purchased were conducted by the same group of researchers using samples from low-income communities in New York, NY and Newark, NJ,19 and research has shown that there are socioeconomic disparities in calorie label use, with higher-income individuals being more likely to notice calorie labels.20 Another study limited its sample population to one chain of restaurants in King County, WA.21 An additional factor to consider is the time frame between implementation of menu labeling and an assessment of purchasing behavior, as there needs to be enough time for an effect to take place. One study, for instance, did not find an effect at four to six months post-mandatory menu labeling, but it did find a decrease in calories purchased 18 months after implementation.22 Another study that did not find an effect of menu labeling on calories purchased examined outcomes two months post implementation, which may not have been enough time for an effect to take place.23 In addition, most of these studies relied on self-reported data to assess customers' awareness and use of calorie labels. Such self-reporting may not be accurate, as evidenced by the inconsistencies between reported calories purchased and actual calories purchased as indicated on receipts.24 Finally, these studies analyzed the number of calories purchased but not changes in calories consumed, which may differ in response to menu labeling. For example, in full-service restaurants, customers may be more likely to share a meal or eat half the meal and take the rest home, which would not be captured by transaction data. Similarly, in fast food or carry-out establishments, customers may consume only a portion of their meal, which would not be captured by transaction data.
Studies of the Impact of Menu Labeling on Sales and Revenue
In 2009, Starbucks commissioned a Stanford University study to determine how the menu labeling mandate in New York City (NYC) affected its overall sales.25
Findings indicate that after the implementation of mandatory calorie labeling, average calories per transaction fell by 6% at Starbucks, an effect that lasted 10 months after the calorie posting commenced. This effect was primarily found for food purchases, as the average food calories per transaction fell by 14% (i.e., approximately 14 calories per transaction), while average beverage calories per transaction did not substantially change. Changes in beverage calories may not be reflected in transaction data. For example, if a customer orders a latte and substitutes skim milk for 2% milk, or asks for one pump of syrup instead of the usual three or four, those substitutions would not be captured by transaction data because the cost of the latte would not change.
This study also assessed the impact of calorie posting on Starbucks revenue, reporting no statistically significant change in revenue as a result of calorie labeling. Because cost data associated with the policy was unavailable, profits were not measured directly. The effect on revenue was divided into (1) the effect on the number of transactions and (2) the effect on revenue per transaction. The study found that daily store transactions increased by 1.4% on average, while revenue per transaction decreased by 0.8% on average for all Starbucks in NYC, resulting in a zero net impact of calorie posting on Starbucks revenues. In NYC Starbucks stores located within 100 meters of a Dunkin Donuts, daily revenue increased by 3.3% on average.
To determine consumers' preliminary knowledge of calories in Starbucks food and beverages, surveys were administered before and after the introduction of a calorie-posting law in Seattle.26 Pre-menu labeling survey data indicate that Starbucks customers tended to be inaccurate in predicting the number of calories in their beverage and food orders. Specifically, in this study, consumers overestimated the number of calories in beverages and underestimated the number of calories in food. This is consistent with the study's finding that calorie posting discouraged individuals from purchasing food but not beverages. Because consumers tended to underestimate the number of calories in food items, seeing the posted caloric value, which was greater than initially expected, may have led consumers to reduce their food purchases. However, because consumers tended to overestimate beverage calories, calorie posting may not have discouraged people from purchasing beverages.
Proponents of menu labeling argue that, in addition to affecting consumer purchasing behavior, mandatory menu labeling may incentivize restaurants to offer lower calorie options and provide consumers with healthier choices. A study in the American Journal of Preventive Medicine reported that new menu items in restaurant chains in 2013 contained approximately 60 fewer calories compared with menu items in 2012—a 12% drop in calories.27 This voluntary action by large chain restaurants may have been in anticipation of the ACA's federal menu-labeling provisions which will be in effect May 7, 2018.
FDA's Authority to Regulate Nutrition Labeling
The Federal Food, Drug, and Cosmetic Act of 1938 authorized FDA to regulate most food products and their ingredients. In 1990, Congress passed the Nutrition Labeling and Education Act (NLEA P.L. 101-535), which amended the FFDCA and gave FDA authority to require nutrition labeling of most foods (including dietary supplements), exempting restaurants from this requirement. However, current regulations pursuant to the NLEA requirement do require restaurants and SRFEs that make either a nutrient content or health claim to provide certain nutrition information upon request. For example, if an entrée is listed as low-fat, the restaurant must be able to provide information about the fat content of the entrée upon request.28
Section 4205 of the Affordable Care Act (P.L. 111-148, ACA) amended FFDCA Section 403 to establish nutrition labeling requirements for standard menu items offered for sale in chain restaurants or SRFEs that have 20 or more locations, that conduct business under the same name regardless of the type of ownership of the locations, and that offer the same menu items for sale.29 Establishments subject to the menu labeling requirements must disclose the number of calories in each item "as usually prepared and offered for sale," and must post a succinct statement, specified by regulation, concerning suggested daily caloric intake on menus and menu boards. The law requires covered establishments to provide additional nutrition information (e.g., total fat) to consumers in writing upon request. Self-service food or food on display must have an adjacent sign that lists calories per displayed food item or per serving. Certain food items are exempted from the labeling requirements, such as items not listed on a menu or menu board (e.g., condiments), daily specials and temporary items appearing on the menu for less than 60 days, custom orders, and food items that are part of a market test and on the menu for less than 90 days. Covered establishments must have a "reasonable basis" for their nutrient content disclosures, such as nutrient databases, cookbooks, or laboratory analyses. The Secretary must establish standards for determining and disclosing the nutrient content for standard menu items that come in different flavors, varieties, or combinations but are listed as single menu items (e.g., ice cream, pizza, doughnuts, or children's combination meals).
In FDA's final regulatory impact analysis, the Agency estimated that approximately 298,600 establishments, organized under 2,130 chains, would be covered by the menu labeling regulations (see Table 1).
|
Sector of Industry |
Estimated No. of Chain Retail Food Establishments |
Estimated No. of Associated Chains |
|
Full Service Restaurants and Drinking Places |
115,000 |
530 |
|
Limited Service Restaurants |
116,200 |
540 |
|
Supermarkets and Grocery Stores |
11,200 |
120 |
|
Convenience Stores |
36,200 |
450 |
|
General Merchandise Stores |
3,200 |
90 |
|
Managed Food Services |
4,500 |
50 |
|
Lodging |
6,200 |
100 |
|
Recreation, Sports, and Performing Arts |
3,300 |
200 |
|
Motion picture and video exhibition |
2,800 |
50 |
|
Total Covered |
298,600 |
2,130 |
Sources: "Food Labeling: Nutrition Labeling of Standard Menu Items in Restaurant and Similar Retail Food Establishments," Final Regulatory Impact Analysis FDA-2011-F-0172, p. 33 and U.S. Census Bureau, County Business Patterns, United States NAICS 2000-2008, 2014, 10-18-2010.
Notes: In FDA's analysis, the definition of chain retail food establishments in the final rule is drawn from the industry sectors listed in the table above, as classified by the North American Industry Classification System (NAICS). "The NAICS is the standard used by Federal statistical agencies in classifying business establishments for the purpose of collecting, analyzing, and publishing statistical data related to the U.S. business economy" (http://www.census.gov/eos/www/naics/).
The law also established nutrition labeling requirements for vending machine items and amended FFDCA Section 403A regarding federal preemption of state and local food labeling requirements.30
FDA Rulemaking on Menu Labeling
As noted, FFDCA Section 403(q)(5)(H) requires the Secretary to promulgate regulations to implement the menu labeling requirements, including standards for determining and disclosing the nutrient content for standard menu items that come in different flavors, varieties, or combinations but are listed as single menu items, as well as registration rules for establishments that are not otherwise subject to the law's requirements to voluntarily provide nutrition information. In promulgating regulations, the Secretary is required to consider, among other things, the format and manner of the nutrient content disclosure requirement.31 The Secretary may also require, by regulation, that other nutrient information be disclosed to help consumers maintain healthy dietary practices.32 In 2011, FDA published two proposed rules establishing calorie labeling requirements for food items sold in restaurants and vending machines. The two rules were finalized and published in the Federal Register on December 1, 2014.
This section provides an overview of the FDA's proposed and final regulations on menu labeling, and describes corresponding statutory requirements upon which the regulations are based.
Covered Establishments
To be subject to the menu labeling requirements, a restaurant or SRFE must be part of a chain of 20 or more locations doing business under the same name (regardless of the type of ownership of the locations) and offering for sale substantially the same menu items.33 Restaurants and SRFEs may also voluntarily register with FDA to become subject to the menu labeling regulations. The meaning of SRFE is not defined in statute.
The Proposed Rule
The proposed rule defined restaurant or similar retail food establishment as a retail establishment that offers for sale restaurant or restaurant-type food, where the sale of food is the primary business activity of that establishment.34
FDA had proposed two options for clarifying which restaurants and similar retail food establishments would be covered by the rule. The two definitions would affect different segments of the food industry. Option 1 would generally have exempted entertainment venues (e.g., movie theaters, amusement parks), general merchandise stores with in-house concession stands, hotels, and transportation (e.g., food trucks, trains, airplanes). Option 2 would have generally excluded the entities in option 1, as well as grocery and convenience stores.35
Final Rule
In the final rule, FDA defines a restaurant or similar retail food establishment to mean "a retail establishment that offers for sale restaurant-type food, except if it is a school as defined in 7 CFR 210.2 or 220.2."36 FDA clarified that
Establishments such as bakeries, cafeterias, coffee shops, convenience stores, delicatessens, food service facilities located within entertainment venues (such as amusement parks, bowling alleys, and movie theaters), food service vendors (e.g., ice cream shops and mall cookie counters), food take-out and/or delivery establishments (such as pizza take-out and delivery establishments), grocery stores, retail confectionary stores, superstores, quick service restaurants, and table service restaurants would be restaurants or similar retail food establishments if they sell restaurant-type food.37
Covered entities under the final rule include restaurants and SRFEs with 20 or more locations, as well as supermarkets and convenience stores, and entertainment venues such as bowling alleys and movie theaters. FDA's decision to include supermarkets and convenience stores under the definition of SRFE has generated debate, with some industry representatives questioning FDA's broad interpretation of the statutory provision. Specifically, representatives of the supermarket industry have expressed concern over the scope of the definition of covered establishments, citing that implementing menu labeling would be costly and complex for grocery and convenience stores.38 Unlike many restaurants, retail supermarkets merchandise food in various forms (e.g., service, self-service, cold, hot), and the types of food offered at retail supermarkets can vary throughout the year depending on season, holiday, and promotion. FDA has provided some flexibility, exempting certain foods purchased in retail establishments, such as items that are intended for more than one person to eat (e.g., a loaf of bread, rotisserie chicken) and some items sold at deli counters, such as meat, cheeses, and bulk salads.39
Covered Food
The menu labeling requirements apply to standard menu items offered for sale at covered establishments.40 Certain foods served at covered establishments are exempt from labeling requirements:
- custom orders, which are prepared in a specific manner at the customer's request;
- daily specials—foods that are not routinely listed on the menu;
- temporary menu items, which appear on a menu or menu board for less than 60 days per calendar year;
- market test items—foods that are offered for fewer than 90 consecutive days to test consumer acceptance; and
- condiments available for general use that every customer has access to (e.g., salt, pepper, ketchup).41
Proposed Rule
FDA proposed to define restaurant food as "food that is served in restaurants or other establishments in which food is served for immediate human consumption, i.e., to be consumed either on the premises where that food is purchased or while walking away; or which is sold for sale or use in such establishments."42 Restaurant-type food was proposed to mean "food of the type described in the definition of 'restaurant food' that is ready for human consumption, offered to sale for customers, but not for immediate consumption, processed and prepared primarily in a retail establishment, and not offered for sale outside that establishment."43 The proposed rule further specified which foods would require labeling and which foods would be exempt, including alcoholic beverages.44
Final Rule
In the final rule, FDA did not define restaurant food and revised the definition of restaurant-type food to better reflect the type of food usually offered for sale in restaurants.45 The final rule defines restaurant-type food as
food that is (1) usually eaten on the premises, while walking away, or soon after arriving at another location; and (2) either (i) served in restaurants or other establishments in which food is served for immediate human consumption or which is sold for sale or use in such establishments; or (ii) processed and prepared primarily in a retail establishment, ready for human consumption of the type described in (i), and offered for sale to consumers but not for immediate human consumption in such establishment and which is not offered for sale outside such establishment.46
Foods covered and not covered by the new definition are listed in Table 2.
|
What Is Covered? |
What Is Not Covered? |
|
Restaurants and fast food establishments Bakeries, coffee shops, and restaurant type-foods in grocery and convenience stores Take-out and delivery foods, including pizza Self-serve foods from salad or hot-food bars Alcoholic drinks such as cocktails when they appear on menus Foods at entertainment venues, such as movie theaters, amusement parks and bowling alleys |
Foods sold at deli counters and typically intended for more than one person Bottles of liquor displayed behind a bar Food in transportation vehicle (e.g., food trucks, airplanes, and trains) Food on menus in schools that are part of the USDA school nutrition programs (although vending machines in such locations are covered) |
Source: Table created by CRS based on the FDA page, "How Many Calories? Look at the Menu!," https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm423082.htm.
Menus and Menu Boards
The law defines menu or menu board to mean "the primary writing of the restaurant or other similar retail food establishment from which a customer makes an order selection."47 The law requires that covered establishments provide calorie information on menus and menu boards, and include a statement of availability of other nutrition information upon request.
Proposed Rule
In the proposed rule, FDA defined menu or menu board as the primary writing from which a customer makes an order selection, including but not limited to breakfast, lunch, and dinner menus; dessert menus; beverage menus; children's menus; other specialty menus; electronic menus; and menus on the Internet. This definition includes different menu forms, such as booklets, pamphlets, and single sheets of paper.48 Menu boards may be inside a restaurant or SRFE or outside (e.g., drive-through menu boards). In the proposed rule, FDA tentatively concluded that take-out and delivery menus would be considered within the definition of menus to the extent that they included all or a significant portion of items offered for sale.49
Final Rule
In the final rule, FDA determined that take-out and delivery menus would not be considered primary writing solely on the basis of whether they include all or a significant portion of items offered for sale.50 Instead, FDA identified several other factors that would determine whether certain writing qualifies as the primary writing from which a customer makes a selection. For the purposes of the final rule, a written material would be considered a menu if it includes the name (or image) and price of a standard menu item, as well as if it provides a means for the customer to order from while viewing the writing at the restaurant or SRFE.51
There has been some concern with FDA's interpretation of primary writing. Pizza companies, for example, have argued against in-store menu boards, citing that most of their business is driven by phone and online ordering.52 According to a Domino's Pizza representative, only about 10% of ordering occurs by a customer walking into a Domino's store and selecting an item from the menu board.53 Thus, representatives of the pizza industry propose that pizza companies be allowed to list calorie counts online instead of at their stores.
Convenience store representatives have also expressed concern with FDA's final rule, arguing that the way their stores acquire, prepare, and sell food is very different from chain restaurants. Stores that are part of the same chain, for example, may sell the same food items, but the individual stores often vary in how those items are offered and prepared, as influenced by geographic region and market demand. In addition, grocery and convenience stores offer food in many different settings, such as counter areas, self-service coffee and soda stations, baked goods displayed away from the counter area, and refrigerated "grab-and-go" foods. Many of these foods and beverages are not listed on the menu boards that sometimes appear above the counter.54
Calorie Declaration and Other Nutrition Information
The law requires covered establishments to disclose the number of calories contained in standard menu items.55 Calorie information must have a reasonable basis for its nutrition information disclosures (e.g., nutrient databases, cookbooks, laboratory analyses, and other reasonable means) as described in specified regulations.56 The calorie information must be displayed adjacent to the standard menu item on all menus and menu boards. The restaurant or SRFE must also provide a succinct statement, as specified by regulation, concerning daily recommended caloric intake to inform the consumer of the significance of the standard menu item in the context of a daily diet. The covered establishment must also be able to provide additional nutrition information, in written form, upon the consumer's request.57
Proposed Rule
FDA proposed the following succinct statement to be listed on menus and menu boards:
A 2,000 calorie daily diet is used as the basis for general nutrition advice; however, individual calorie needs may vary.58
Per the proposed rule, calories would have to be declared to the nearest 5-calorie increment in foods containing 50 calories or less, and to the nearest 10-calorie increment in foods containing more than 50 calories; and the term "Calories" or "Cal" would have to appear as a heading above a column listing the number of calories for each standard menu item, or adjacent to the number of calories for each standard menu item.59
FDA proposed use of the "80/120 rule" for nutrient substantiation, which would permit a narrow deviation between the posted calorie value for a particular item and the actual calorie content of the item.60 This aspect of the rule was modeled after regulations for prepackaged foods. Several comments on the proposed rule opposed use of the 80/120 standard, asserting that restaurant food is generally prepared via human labor, and is thereby subject to wider variation. For example, adding seven French fries to an order could increase calories by more than 20%, or an extra squirt of mayonnaise could render the nutrient content declaration out of compliance, deeming the food product misbranded under the "80/120 rule."61
Final Rule
The final rule requires the following statement to be included on the menu or menu board:
2,000 calories a day is used for general nutrition advice, but calorie needs vary.62
Calorie information must be displayed adjacent to the name of the standard menu item on all menus and menu boards, as specified; calories must be declared to the nearest 5-calorie increment in foods containing 50 calories or less, and to the nearest 10-calorie increment in foods containing more than 50 calories; and the term "Calories" or "Cal" must appear as a heading above a column listing the number of calories for each standard menu item, or adjacent to the number of calories for each standard menu item.63 The menu or menu board must contain the statement, "Additional nutrition information available upon request."64
In the case of multiple-serving standard menu items, the calorie declaration must be for the whole menu item (e.g., pizza: 1,600 calories) or per serving, as long as the number of servings is listed on the menu as well (e.g., pizza: 200 cal./slice, 8 slices).65 Additional information regarding FDA's formatting requirements for calorie declaration on menu and menu boards can be found in FDA regulations, guidance, and resources for industry.66
In the final rule, FDA determined that using the "80/120 rule" for establishing compliance with the nutrition labeling requirements would raise practical problems. Instead, FDA specified that nutrient declarations must be accurate and consistent with the scientific basis used to determine values.67 In addition, covered establishments are required to submit to FDA, upon request, information substantiating nutrient values. If a nutrient database is used as the method of reasonable basis, for example, certain information must be provided to FDA, such as the identity of the database used; the recipe or formula used as a basis for the nutrient declarations; a detailed listing of the amount of each nutrient that that ingredient contributes to the menu item; and, among other information, a statement signed by a responsible employee of the covered establishment certifying that the provided information is complete and accurate.68
Industry groups have argued that FDA's final rule contains rigid calorie labeling requirements, and have asked for labeling flexibility that would permit establishments to provide calorie information in the form of ranges, averages, or standard offerings (e.g., the information for a sandwich without regard to whether the customer orders extra cheese or condiments), among other methods.69 In grocery stores, for example, foods that are "packaged and prepared for immediate consumption" are not always pre-portioned (e.g., salad bar or hot food bar items) and would not be served in standardized sizes. In addition, certain items in food establishments are served as a whole (e.g., pizza), with a variety of food combinations possible. According to the testimony of a Domino's Pizza representative, based on various combinations of crust types, sauces, and toppings, there are over 34 million ways to make a pizza at Domino's and 2 billion ways at Pizza Hut. These combinations make it difficult, if not impossible, to list all the iterations of pizza types on a menu board.70
FDA Rulemaking on Vending Machine Labeling
In addition to the requirements for restaurants and SRFEs, the FFDCA also requires calorie labeling for food sold from vending machines; specifically, operators who own or operate 20 or more vending machines must disclose calorie information for food sold from vending machines, subject to certain exemptions.71 In tandem with the restaurant menu labeling rule, FDA issued a final rule regarding calorie labeling for food items sold in covered vending machines.72 Vending machine operators who are not subject to the calorie labeling requirements may voluntarily register with FDA to be covered by the regulation.
The final rule defines a vending machine as "a self-service machine that, upon insertion of a coin, paper currency, token, card, or key, or by optional manual operation, dispense servings of food in bulk or in packages or prepared by the machine, without the necessity of replenishing the machine between each vending operation."73 The rule requires that if a vending machine does not permit a consumer to examine nutrition information before purchase or at point-of-purchase, then the vending machine operator must provide calorie declarations for such foods via a sign close to the article of food or on a selection button (i.e., in, on, or adjacent to the vending machine).74
Compliance and Enforcement
As previously noted, in 2011, FDA published two proposed rules establishing calorie labeling requirements for food items sold in restaurants and vending machines. The two rules were finalized and published in the Federal Register on December 1, 2014, and were to take effect one year from publication (December 1, 2015) for restaurants and two years (December 1, 2016) for vending machines.75 On July 9, 2015, FDA extended the compliance date until December 1, 2016, for restaurants and SRFEs.76 Compliance with the regulations was delayed again as a result of language included in the Consolidated Appropriations Act of 2016 (P.L. 114-113), which prohibited the use of any funds for implementation, administration, or enforcement of the menu labeling requirements until the later of December 1, 2016, or until one year from the date that the Secretary of the Department of Health and Human Services (HHS) issues final, Level 1 guidance on compliance with specified requirements for menu labeling contained in the final menu labeling rule. FDA issued draft guidance to help companies comply with the menu labeling final rule on September 11, 2015, and final guidance on May 5, 2016. In issuing the final guidance, FDA announced that enforcement of the final rule would commence on May 5, 2017.77 However, in response to continued concerns from certain sectors of the affected industry and some Members of Congress, FDA announced that it was further extending the compliance date to May 7, 2018.78
FDA has also extended the compliance date for calorie labeling of certain food products sold in vending machines to July 26, 2018.79
A standard menu item offered for sale in a covered establishment would be deemed misbranded under FFDCA Section 403 if its labeling does not meet the requirements of the final rule.80 For example, if the calorie declaration of a self-service standard menu item or food on display is not listed clearly and conspicuously in compliance with the final rule, that standard menu item would be deemed misbranded.81 Generally, FDA relies on manufacturers to voluntarily recall misbranded products, either by their own initiative or upon regulators' request. However, the Food Safety and Modernization Act (FSMA, P.L. 111-353) provided FDA with mandatory recall authority. In addition, the agency has the authority to pursue other enforcement actions, including warning letters, seizures, injunctions, civil monetary penalties, and prosecution.
Costs and Benefits
FDA estimates that the labeling requirements (both menu and vending machine rules combined) are estimated to have benefits exceeding costs by $477.9 million on an annualized basis (over 20 years discounted at 7%).82
Costs for Restaurants and SRFEs
FDA expects that the final rules would have costs to both industry and consumers. For industry, there will be initial costs associated with implementing the rules (e.g., nutrient content analysis, purchasing menu boards), as well as recurring costs (e.g., employee training). The major elements of cost expected to be incurred by industry include (1) collecting and managing records of nutritional analysis for each food item subject to the labeling requirement; (2) revising and replacing existing menus and menu boards, and providing written nutrition information; (3) training employees to understand nutrition information; and (4) legal review.
Cost of Nutrition Analysis
Cost estimates for nutrition analysis vary depending on several factors, such as the complexity of the food item, detail of the nutrition report, and whether the analysis is conducted using existing databases or using item-specific laboratory testing. Most of the cost variation comes from how heavily restaurant chains rely on database analysis versus laboratory testing.83
Cost of Menu Replacement
To comply with FDA regulations, restaurants and SRFEs will be required to replace their existing menus and menu boards with those that list calorie information for standard items. FDA estimates that average menu printing costs would be about $1 to $3 per copy, and the number of menus per establishment is highly variable. FDA estimates the cost for replacing menu boards would be approximately $550 per board.84
Cost of Training
Although not mandated by the final rule, FDA expects that some employee training will be required to ensure that employees are able to respond to consumer questions and to ensure that displayed calorie and nutrition information is in compliance with the final rule.85
Cost of Legal Review
FDA estimates that a legal analyst will spend 8 to 12 hours, on average, learning about the menu labeling rule requirements. At a labor cost of $96 per hour, the estimated cumulative cost of legal review ranges from $1.6 million to $2.5 million.86
Other Costs
A cost not included in FDA's estimate is that associated with reformulating current food items and introducing new food items. Although not required by the rule, there may be incentive for some restaurant chains or SRFEs to create and introduce new, lower-calorie items. In addition, the expense of complying with the final labeling regulations may result in a price increase in the affected food items, potentially resulting in higher costs for consumers.87
Total Costs
In the Final Regulatory Impact Analysis, FDA estimates that approximately 298,600 covered establishments, organized under 2,130 chains, would be affected by the menu labeling rule requirements. FDA estimates an initial cost of $397.03 million and a recurring cost of $55.13 million for complying with the regulations. Annualized over 20 years, the estimated annual cost of the final requirements is $76.90 million (at 3% discount rate) and $84.50 million (at 7% discount rate). Table 3 shows the total estimated costs of the final requirements.88
|
Sector |
Low |
Mean |
High |
Proportion of Costs |
|
Initial Costs |
||||
|
Restaurants |
$223.91 |
$283.22 |
$342.03 |
72% |
|
Grocery, Convenience Store, & |
$70.87 |
$85.02 |
$99.07 |
21% |
|
Managed Food Service |
$5.97 |
$8.86 |
$11.76 |
2% |
|
Lodging |
$1.97 |
$4.66 |
$7.22 |
1% |
|
Sports, Recreation, & Entertainment |
$8.99 |
$15.27 |
$21.51 |
3% |
|
Initial Costs Subtotal |
$311.71 |
$397.03 |
$481.59 |
100% |
|
Annually Recurring Costs Subtotal |
$28.41 |
$55.13 |
$81.55 |
|
|
Total Final Rule Annualized Costs |
||||
|
Annualized @ 3% |
$46.91 |
$76.90 |
$106.56 |
|
|
Annualized @ 7% |
$53.38 |
$84.50 |
$115.28 |
Source: "Food Labeling: Nutrition Labeling of Standard Menu Items in Restaurant and Similar Retail Food Establishments," Final Regulatory Impact Analysis FDA-2011-F-0172, November 2014, https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/LabelingNutrition/UCM423985.pdf.
Notes: When conducting a regulatory impact analysis, an agency should generally construct a range of values for possible outcomes including a "high" and a "low" scenario that provide plausible upper and lower bounds. In this table, "low" refers to the lower boundary of FDA's estimate and "high" refers to the upper boundary. "Mean" refers to a rounded average estimate of the cost.
Costs for Vending Machine Operators
FDA estimates that the total number of operators operating 20 or more vending machines ranges from 8,983 to 11,960, and the total number of associated vending machines (excluding non-food machines) ranges from 4.97 million to 5.98 million.89 For a breakdown of the costs associated with vending machine labeling, see Table 4.
|
Low |
Mean |
High |
|
|
Initial Calorie Analysis |
$0.3 |
$0.5 |
$0.8 |
|
First Year Sign Costs |
$38.9 |
$63.6 |
$110.5 |
|
Bulk Signage |
$.14 |
$.24 |
$.35 |
|
Legal Review |
$5.2 |
$7.0 |
$9.2 |
|
Total Initial Costs |
$39.2 |
$64.2 |
$111.6 |
|
Total Annual Recurring Costs |
$14.5 |
$32.6 |
$72.4 |
|
20-year Present Discounted Value (3%) |
$246.9 |
$531.1 |
$1,148.6 |
|
20-year Present Discounted Value (7%) |
$189.1 |
$401.1 |
$859.9 |
|
Annualized @ 3% |
$16.1 |
$34.7 |
$75.0 |
|
Annualized @ 7% |
$16.7 |
$35.4 |
$75.8 |
Source: "Food Labeling: Calorie Labeling of Articles of Food in Vending Machines," Regulatory Impact AnalysisFDA-2011-F-0171, November 2014, http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/EconomicAnalyses/UCM425973.pdf.
Benefits
National data reveal that approximately two-thirds of the U.S. population is overweight or obese, and a major risk factor for overweight and obesity is overconsumption of calories. The predicted benefits from the labeling regulations stem from the idea that providing consumers with nutrition information at the point of purchase will facilitate informed and healthful dietary choices, which in turn may reduce caloric intake and obesity in the U.S. population.90
The benefit estimates are contingent on several assumptions, including
- increased awareness regarding the caloric content of foods, which would encourage consumption of lower-calorie options, and
- increased consumer interest in lower-calorie options, which would incentivize
- reformulation of current menu items to make them lower calorie or decrease portion sizes, and
- introduction of new lower-calorie items.
Determining the value of menu labeling is difficult because the benefits largely depend on whether or not individuals shift their consumption patterns toward a healthier diet. Studies examining the impact of menu labeling on calories purchased show mixed findings, suggesting that providing consumers with nutrition information does not mean they will make more healthful decisions (see "Research Evaluating the Impact of Menu Labeling"). Further research on this topic, once the labeling rules are in effect, may help determine what impact menu labeling has on consumer purchasing behaviors, if any.
Issues for Congress
In response to concerns from certain sectors of the industry affected by the menu labeling rule, some Members of Congress have supported amending nutrition disclosure requirements to provide for added flexibility.91 For example, as introduced in the 115th Congress, the Common Sense Nutrition Disclosure Act (H.R. 772, S. 261) would permit entities that receive the majority of orders from customers who are off-premises (e.g., pizza chains) to provide their menus online in place of menu boards in restaurants; would give manufacturers greater flexibility in how they determine and document nutrient content analyses; and would permit establishments with standard menu items that come in different flavors, varieties, or combinations that are listed as a single menu item to determine and disclose nutritional information using specified methods or methods allowed by the Secretary.92 Under this proposal, if a restaurant or retailer is determined to be in violation of the menu labeling requirements, the entity would have 90 days to take corrective action, and the Secretary would be prohibited from taking enforcement action if the violations are corrected within those 90 days. In addition, the Secretary would be required to promulgate regulations to carry out the standards for determining and disclosing the nutrient content for standard menu items that come in different flavors, varieties, or combinations, but which are listed as a single menu item. Such regulations, as well as any regulations issued before enactment of the Common Sense Nutrition Disclosure Act, would not be allowed to take effect until the compliance date specified in the final regulations promulgated pursuant to this Act.
As mentioned, the menu labeling compliance date has been extended multiple times since the final rule was issued in December 2014. Opponents of the extension have argued that many chains are successfully complying with the labeling requirements and that consumers want menu labeling. Several public health groups have signed onto a letter to FDA opposing changes to the menu labeling requirements.93