FDA Overview
The Food and Drug Administration (FDA) regulates the safety of foods (including dietary supplements), cosmetics, and radiation-emitting products; the safety and effectiveness of drugs, biologics (e.g., vaccines), and medical devices; and public health aspects of tobacco products.1 Although FDA has been a part of the Department of Health and Human Services (HHS) since 1940, the Committees on Appropriations do not consider FDA within the rest of HHS under their Subcommittees on Labor, Health and Human Services, and Education, and Related Agencies. Jurisdiction over FDA's budget remains with the Subcommittees on Agriculture, Rural Development, Food and Drug Administration, and Related Agencies, reflecting FDA's beginnings as part of the Department of Agriculture.
|
FDA Centers Center for Biologics Evaluation and Research (CBER) Center for Devices and Radiological Health (CDRH) Center for Drug Evaluation and Research (CDER) Center for Food Safety and Applied Nutrition (CFSAN) Center for Tobacco Products (CTP) Center for Veterinary Medicine (CVM) National Center for Toxicological Research (NCTR) |
Seven centers within FDA represent the broad program areas for which the agency has responsibility, along with various other offices that have agency-wide responsibilities. Table 1 is organized in a format consistent with the Administration's budget request as presented in the FDA Congressional Justification, as well as with the materials of the Committees on Appropriations—each program area includes funding (FY2013-FY2017, as well as the Administration's FY2018 request) designated for the responsible FDA center (e.g., CDER or CFSAN) and the portion of funding for the FDA-wide Office of Regulatory Affairs that is committed to that program area.
Funding Sources
FDA's total program level, the amount that FDA can spend, is composed of direct appropriations (also referred to as budget authority) and user fees.2 In FDA's annual appropriation, Congress sets both the amount of appropriated funds and the amount of user fees that the agency is authorized to collect and obligate for that fiscal year. Appropriated funds are largely for the Salaries and Expenses account, with a much smaller amount for the Buildings and Facilities account. The different user fees contribute only to the Salaries and Expenses account.
The largest and oldest FDA user fee that is linked to a specific program was first authorized by the Prescription Drug User Fee Act (PDUFA, P.L. 102-571) in 1992. After PDUFA, Congress added user fee authorities regarding medical devices, animal drugs, animal generic drugs, tobacco products, priority review, food reinspection, food recall, voluntary qualified food importer, generic drugs, biosimilars, and, most recently, outsourcing facilities (related to drug compounding) and some wholesale distributors and third-party logistics providers (related to pharmaceutical supply chain security).3 Each of the medical product fee authorities requires reauthorization every five years. Several indefinite authorities apply to fees for mammography inspection, color additive certification, export certification, priority review vouchers, food and feed recall, food inspection, voluntary qualified food importers, third-party auditor, and outsourcing facilities.
FDA Recent Funding History and the FY2017 Omnibus
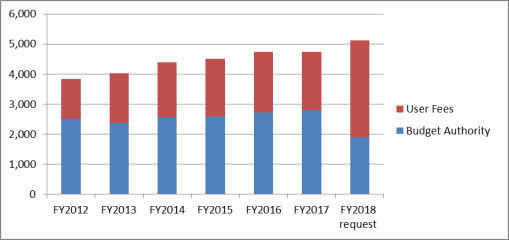
Between FY2012 and FY2017, FDA's total program level increased from $3.832 billion to $4.745 billion. Although congressionally appropriated funding increased by 11% over that time period, user fee revenue increased more than 47%. In FY2017, user fees account for 41% of FDA's total program level. Under the Trump Administration's FY2018 request, user fees would account for 63% of the FDA's total program level.4
|
Figure 1. FDA Budget, by Source, FY2012-FY2018 (in millions of dollars) |
 |
|
Source: FY2012 amounts are from the FY2013 Sequestration Operating Plan. FY2013 and FY2014 amounts are from the FDA FY2014 Operating Plan. FY2013 figures reflect sequestration. The enacted FY2015 and FY2016 amounts are from the FDA FY2016 Operating Plan. The FY2017 amounts are from the 2017 Consolidated Appropriations Act (P.L. 115-31) and the accompanying Explanatory Statement, as well as the 2017 Further Continuing and Security Assistance Appropriations Act (P.L. 114-254). The FY2018 request amounts are from the FY2018 FDA Justification of Estimates for Appropriations Committees, https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/BudgetReports/UCM559923.pdf. |
The Administration's FY2018 request includes a total program level of $5.112 billion, an increase of $367 million (+8%) over the FY2017 enacted amount.5 The FY2018 request proposes $1.888 billion in direct appropriations—a decrease of $903 million (-32%) from the FY2017 enacted amount. The FY2018 request includes in the direct appropriation $60 million from the FDA Innovation Account, which was established by Section 1002 of the 21st Century Cures Act (P.L. 114-255).6
For user fees, the FY2018 request proposes $3.223 billion in fees—an increase of $1.269 billion or 65% over the FY2017 enacted amount—to be collected through authorized programs to support specified agency activities regarding prescription drugs, medical devices, animal drugs, animal generic drugs, tobacco products, generic human drugs, biosimilars, mammography quality, color certification, export certification, food reinspection, food recall, the voluntary qualified importer program, outsourcing facilities, priority review vouchers, and third-party auditors. In addition to the $3.223 billion in user fees from currently authorized programs, the Administration requests for FY2018 an additional $4.28 million in as yet unauthorized fees to support export certification activities. With those proposed fees, the Administration's total user fee request comes to $3.228 billion, bringing the program level request to $5.116 billion.
Not included in any of these totals is the $10 million (to the Salaries and Expenses account) provided by Section 752 of the FY2017 enacted appropriation for FDA to "prevent, prepare for, and respond to emerging health threats, including the Ebola and Zika viruses, domestically and internationally and to develop necessary medical countermeasures and vaccines, including the review, regulation, and post market surveillance of vaccines and therapies, and for related administrative activities ... to remain available until expended."
The human drugs program comprises the largest portion of FDA's budget (28% in FY2017), followed by the foods program (22% in FY2017), and the tobacco program (13% in FY2017). Table 1 displays, by program area, the budget authority (direct appropriations), user fees, and total program levels for FDA in previous years.
|
Program Area |
FY2012 |
FY2013 |
FY2014 |
FY2015 |
FY2016 |
FY2017 |
FY2018 Request |
|||||||
|
Foods (CFSAN) |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Human drugs (CDER) |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Biologics (CBER) |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Animal drugs and feeds (CVM) |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Devices and radiological health (CDRH) |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Tobacco products (CTP) |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Toxicological research (NCTR) |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Headquarters/ Commissioner's Office |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
GSA rent |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Other rent, rent-related activitiesb |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Export, color certificationc |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Priority review voucher |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Food and Drug Safetyd |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Buildings & Facilities |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
FDA Innovation Accounte |
|
|
|
|
|
|
|
|||||||
|
BA |
|
|
|
|
|
|
|
|||||||
|
Fees |
|
|
|
|
|
|
|
|||||||
|
Total Budget Authority |
|
|
|
|
|
|
|
|||||||
|
Total User Fees |
|
|
|
|
|
|
|
|||||||
|
Total Program Level |
|
|
|
|
|
|
|
Sources: FY2012 amounts are from the FY2013 Sequestration Operating Plan. FY2013 and FY2014 amounts are from the FDA FY2014 Operating Plan. FY2013 figures reflect sequestration. FY2014 amounts are from the FDA FY2014 Operating Plan. The FY2015 and FY2016 amounts are from the FDA FY2016 Operating Plan. The FY2017 amounts are from the 2017 Consolidated Appropriations Act (P.L. 115-31) and its Explanatory Statement (for H.R. 244 in the May 3, 2017, Congressional Record), as well as the 2017 Further Continuing and Security Assistance Appropriations Act (P.L. 114-254). The FY2018 request amounts are from the FY2018 FDA Justification of Estimates for Appropriations Committees.
Notes: Individual amounts may not add to subtotals or totals due to rounding. Consistent with the Administration and congressional committee formats, each program area includes funding designated for the responsible FDA center (e.g., the Center for Drug Evaluation and Research or the Center for Food Safety and Applied Nutrition) and the portion budgeted for agency-wide Office of Regulatory Affairs in that area.
a. P.L. 114-113 (for FY2016) and P.L. 115-31 (for FY2017) required that $1.5 million of the budget authority provided for "other activities" (e.g., Office of the Commissioner) be transferred to the HHS Office of Inspector General for FDA oversight.
b. Other rent and rent-related activities include White Oak consolidation.
c. The FY2014-FY2017 amounts reflect the color certification fees authorized by the Color Additive Amendments of 1960 (P.L. 86-618) and export certification for medical products authorized by the FDA Export Reform and Enhancement Act of 1996 (P.L. 104-134). The Food Safety Modernization Act (FSMA) of 2011 (P.L. 111-353) authorized FDA to collect export certification fees also for food. Note that the Appropriations Committees have not included funding for the export certification fees authorized by FSMA.
d. The FY2013 Sequestration Operating Plan notes food safety and drug safety items that had not been included in the program-level appropriations. Subsequent years' bills have not specified this distinct item.
e. In December 2016, Congress passed a measure providing continuing appropriations for FY2017 through April 28, 2017 (P.L. 114-254). The law provided to the FDA an additional $20 million for FY2017, pursuant to the 21st Century Cures Act (P.L. 114-255), which establishes an FDA Innovation Account to help fund the agency's activities and programs authorized in Division A of the Cures Act (e.g., changes to the drug and device FDA approval pathways).
f. Table VIII of P.L. 113-235 (for FY2015) provided an additional, one-time $25 million in direct appropriations to FDA for Ebola response and preparedness activities. Adding this $25 million to the FDA appropriations made in Title VI brought BA to $2.622 billion and the total program level to $4.525 billion for FY2015.
g. For user fees in the Administration's FY2018 request, this column shows only the $3.223 billion in fees that have been authorized. The request included $4.2 million in proposed additional export certification fees. With these proposed user fees, the FY2018 request for user fees totals $3.228 billion yielding a total program level request of $5.116 billion.
h. Section 752 provides an additional $10 million for FDA to "prevent, prepare for, and respond to emerging health threats...." Adding this $10 million to the FDA appropriations brings BA to $2.801 billion and the total program level to $4.755 billion for FY2017.